Abstract
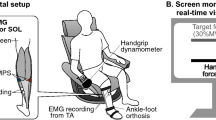
A multitude of studies have demonstrated a clear activation of the motor cortex during imagination of various motor tasks; however, it is still unclear if movement-related parameters (movement direction, range of motion, speed, force level and rate of force development) specifically modulate cortical activation as they do during the execution of actual motor tasks. Accordingly, this study examined whether the rate of torque development (RTD) and/or the torque amplitude modulates cortical potentials generated during imaginary motor tasks. Fifteen subjects imagined four different left-sided isometric plantar-flexion tasks, while EEG and EMG recordings were being performed. The averaged EEG activity was analyzed in terms of movement-related potentials (MRPs), consisting of readiness potential (RP), motor potential (MP) and movement-monitoring potential (MMP). It was demonstrated that RTD and torque amplitude indeed modulate cortical activity during imaginary motor tasks. Information concerning movement-related parameters for imaginary plantar-flexion tasks seems to be encoded in the supplementary motor area (SMA) and the primary motor cortex (M1). A comparison between MRPs of imaginary and actual motor tasks revealed that early MRPs were morphologically similar, but differed significantly in amplitude. One of the possible suggestions to explain such a difference may be an “abortion” of ongoing motor programs.





Similar content being viewed by others
References
Agnew JA, Zeffiro TA, Eden GF (2004) Left hemisphere specialization for the control of voluntary movement rate. Neuroimage 22(1):289–303
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
American Electroencephalographic Society (1994) Guideline thirteen: guidelines for standard electrode position nomenclature. J Clin Neurophysiol 11:111–113
Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS (1993) Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med 30(2):161–173
Beisteiner R, Hollinger P, Lindinger G, Lang W, Berthoz A (1995) Mental representations of movements. Brain potentials associated with imagination of hand movements. Electroencephalogr Clin Neurophysiol 96(2):183–193
Bonnet M, Decety J, Jeannerod M, Requin J (1997) Mental simulation of an action modulates the excitability of spinal reflex pathways in man. Brain Res Cogn Brain Res 5:221–228
Brunia CH, Van den Bosch WE (1984) Movement-related slow potentials. I. A contrast between finger and foot movements in right-handed subjects. Electroencephalogr Clin Neurophysiol 57:515–527
Brunia CH, Voom FJ, Berger MP (1985) Movement related slow potentials. II. A contrast between finger and foot movements in left-handed subjects. Electroencephalogr Clin Neurophysiol 60:135–145
Cunnington R, Iansek R, Bradshaw JL, Phillips JG (1996) Movement-related potentials associated with movement preparation and motor imagery. Exp Brain Res 111:429–436
Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH (2001) Relationship between muscle output and functional MRI-measured brain activation. Exp Brain Res 140(3):290–300
Decety J, Grèzes J (1999) Neural mechanisms subserving the perception of human actions. Trends Cogn Sci 3(5):172–178
Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS (1991) Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84(2):393–402
Deiber MP, Ibanez V, Sadato N, Hallett M (1996) Cerebral structures participating in motor preparation in humans: a positron emission tomography study. J Neurophysiol 75:233–247
Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M (1999) Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 81(6):3065–3077
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. J Neurosci Methods 134:9–21
van Dijk JH (1979) A theory on the control of arbitrary movements. Biol Cybern 32(4):187–199
Ehrsson HH, Naito E, Geyer S, Amunts K, Zilles K, Forssberg H, Roland PE (2000) Simultaneous movements of upper and lower limbs are coordinated by motor representations that are shared by both limbs: a PET study. Eur J Neurosci 12:3385–3398
Ersland L, Rosen G, Lundervold A, Smievoll AI, Tillung T, Sundberg H, Hugdahl K (1996) Phantom limb imaginary fingertapping causes primary motor cortex activation: an fMRI study. Neuroreport 8(1):207–210
Fattapposta F, Pierelli F, My F, Mostarda M, Del Monte S, Parisi L, Serrao M, Morocutti A, Amabile G (2002) L-dopa effects on preprogramming and control activity in a skilled motor act in Parkinson’s disease. Clin Neurophysiol 113(2):243–253
Ghez C, Krakauer J (2000) The organization of movement. In: Kandel ER, Schwartz JH, Jessell TM (eds) Principles of neural science. McGraw-Hill, New York, pp 653–673
Hink RF, Deecke L, Kornhuber HH (1983) Force uncertainty of voluntary movement and human movement-related potentials. Biol Psychol 16(3–4):197–210
Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ (2000) Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123(Pt 6):1216–1228
Juul PR, Ladouceur M, Nielsen KD (2000) Coding of lower limb muscle force generation in associated EEG movement related potentials: preliminary studies toward a feed-forward control of FES-assisted walking. In: Sinkjær T, Popovic D, Struijk JJ. Aalborg University, Denmark, pp 335–337. Conference proceeding
Klem GH, Lüders HO, Jasper HH, Elger C (1999) The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol Suppl 52:3–6
Lacourse MG, Cohen MJ, Lawrence KE, Romero DH (1999) Cortical potentials during imagined movements in individuals with chronic spinal cord injuries. Behav Brain Res 104(1–2):73–88
Lee KM, Chang KH, Roh JK (1999) Subregions within the supplementary motor area activated at different stages of movement preparation and execution. Neuroimage 9:117–123
Maruno N, Kaminaga T, Mikami M, Furui S (2000) Activation of supplementary motor area during imaginary movement of phantom toes. Neurorehabil Neural Repair 14(4):345–349
Masaki H, Takasawa N, Yamazaki K (1998) Enhanced negative slope of the readiness potential preceding a target force production task. Electroencephalogr Clin Neurophysiol 108(4):390–397
do Nascimento OF, Nielsen KD, Voigt M (2005) Relationship between plantar-flexor torque generation and the magnitude of the movement-related potentials. Exp Brain Res 160:154–165
Pfurtscheller G, Neuper C (2001) Motor imagery and direct brain–computer communication. Proc IEEE 89(7):1123–1134
Porro CA, Cettolo V, Francescato MP, Baraldi P (2000) Ipsilateral involvement of primary motor cortex during motor imagery. Eur J Neurosci 12(8):3059–3063
Ranganathan VK, Siemionow V, Sahgal V, Liu JZ, Sahgal V, Yue GH (2004) From mental power to muscle power—gaining strength by using the mind. Neuropsychologia 42:944–956
Rijntjes M, Dettmers C, Buchel C, Kiebel S, Frackowiak RS, Weiller C (1999) A blueprint for movement: functional and anatomical representations in the human motor system. J Neurosci 19:8043–8048
Romero DH, Lacourse MG, Lawrence KE, Schandler S, Cohen MJ (2000) Event-related potentials as a function of movement parameter variations during motor imagery and isometric action. Behav Brain Res 117(1–2):83–96
Siemionow V, Yue GH, Ranganathan VK, Liu JZ, Sahgal V (2000) Relationship between motor activity-related cortical potential and voluntary muscle activation. Exp Brain Res 133(3):303–311
Siemionow V, Fang Y, Sahgal V, Boros J, Yue GH (2002) Relationship between motor activity-related cortical potential and lower extremity muscle activation, Program No. 366.1, Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington. Online
Siemionow V, Boros J, Fang Y, Yao B, Liu JZ, Sahgal V, Yue GH (2003) Linear frequency modulation of EEG signals during voluntary activation of human lower extremity muscles. Program No. 708.4, Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington. Online
Slobounov S, Tutwiler R, Rearick M, Challis JH (1999) EEG correlates of finger movements with different inertial load conditions as revealed by averaging techniques. Clin Neurophysiol 110:1764–1773
Slobounov S, Rearick M, Chiang H (2000a) EEG correlates of finger movements as a function of range of motion and pre-loading conditions. Clin Neurophysiol 111:1997–2007
Slobounov SM, Rearick MP, Simon RF, Johnston JA (2000b) Movement-related potentials are task or end-effector dependent: evidence from a multifinger experiment. Exp Brain Res 135:106–116
Slobounov S, Johnston J, Chiang H, Ray W (2002) Movement-related EEG potentials are force or end-effector dependent: evidence from a multi-finger experiment. Clin Neurophysiol 113:1125–1135
Spence SA, Frith CD (1999) Towards a functional anatomy of volition. J Conscious Stud 6:11–29
Yom-Tov E, Grossman A, Inbar GF (2001) Movement-related potentials during the performance of a motor task I: the effect of learning and force. Biol Cybern 85:395–399
Acknowledgements
The authors want to thank the financial support provided by Vale do Paraíba University (UNIVAP) and Center for Sensory-Motor Interaction (SMI) from the Department of Health Science & Technology (HST) at Aalborg University (AAU).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nascimento, O.F.d., Nielsen, K.D. & Voigt, M. Movement-related parameters modulate cortical activity during imaginary isometric plantar-flexions. Exp Brain Res 171, 78–90 (2006). https://doi.org/10.1007/s00221-005-0247-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0247-z