Abstract
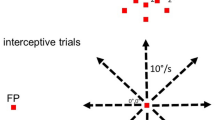
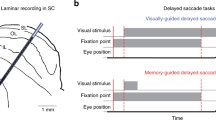
There is significant controversy regarding the ability of the primate visual system to construct stable percepts from a never-ending stream of brief fixations and rapid saccadic eye movements. In this study, we examined the timing and occurrence of perisaccadic modulation of LGN single-unit activity in awake-behaving macaque monkeys while they made spontaneous saccades in the dark and made visually guided saccades to discrete stimuli located outside the receptive field. Our hypothesis was that the activity of LGN cells is modulated by efference copies of motor plans to produce saccadic eye movements and that this modulation depends neither on the presence of feedforward visual information nor on a corollary discharge of signals directing saccadic eye movements. On average, 25% of LGN cells demonstrated significant perisaccadic modulation. This modulation consisted of a moderate suppression of activity that began more than 100 ms prior to the initiation of a saccadic eye movement and continued beyond the termination of the saccadic eye movement. This suppression was followed by a large enhancement of activity after the eyes arrived at the next fixation. Although members of all three LGN relay cell classes (magnocellular, parvocellular, and koniocellular) demonstrated significant saccade-related suppression and enhancement of activity, more cells demonstrated postsaccadic enhancement (25%) than perisaccadic suppression (17%). In no case did the timing of the modulation coincide directly with saccade duration. The degree of modulation observed did not vary with LGN cell class, LGN receptive field center location, center sign (ON-center or OFF-center), or saccade latency or velocity. The time course of modulation did, however, vary with saccade size such that suppression was longer for longer saccades. The fact that activity from a percentage of LGN cells from all cell classes was modulated in relationship to saccadic eye movements in the absence of direct visual stimulation suggests that this modulation is a general phenomenon not tied to specific types of visual stimuli. Similarly, because the onset of the modulation preceded eye movements by more than 100 ms, it is likely that this modulation reflects higher order motor-planning rather than a corollary of mechanisms in direct control of eye movements themselves. Finally, the fact that the largest modulation is a postsaccadic enhancement of activity may suggest that perisaccadic modulations are designed more for the facilitation of visual information processing once the eyes land at a new location than for filtering unwanted visual stimuli.










Similar content being viewed by others
References
Bartlett JR, Doty RW Sr, Lee BB, Sakakura H (1976) Influence of saccadic eye movements on geniculostriate excitability in normal monkeys. Exp Brain Res 25:487–509
Beeler GW Jr (1967) Visual threshold changes resulting from spontaneous saccadic eye movements. Vision Res 7(9):769–775
Bickford M, Ramcharan E, Godwin D, Erisir A, Gnadt J, Sherman S (2000) Neurotransmitters contained in the subcortical extraretinal inputs to the monkey lateral geniculate nucleus. J Comp Neurol 424:701–717
Burr DC, Morrone MC (1996) Temporal impulse response functions for luminance and colour during saccades. Vision Res 36:2069–2078
Burr D, Morrone M, Ross J (1994) Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature 371:511–513
Burr DC, Morgan MJ, Morrone MC (1999) Saccadic suppression precedes visual motion analysis. Curr Biol 9:1207–1209
Buttner U, Fuchs AF (1973) Influence of saccadic eye movements on unit activity in simian lateral geniculate and pregeniculate nuclei. J Neurophysiol 36:127–141
Campbell FW, Wurtz RH (1978) Saccadic omission: why we do not see a grey-out during a saccadic eye movement. Vision Res 18:1297–1303
Casagrande VA, Norton TT (1991) The lateral geniculate nucleus: a review of its physiology and function. In: The Neural Basis of Visual Function, Ed. A.G. Leventhal, Vol. 4 of Vision and Visual Disfunction, ed. J.R. Cronley Dillon, MacMillan, London, pp 41–84
Casagrande VA, Royal DW, Sáry GM (2005) Extraretinal inputs and feedback mechanisms to the lateral geniculate nucleus (LGN). John Wiley and Sons (in press)
Castet E, Masson GS (2000) Motion perception during saccadic eye movements. Nat Neurosci 3:177–183
Derrington AM, Lennie P (1984) Spatial and temporal contrast sensitivities of neurons in lateral geniculate nucleus of macaque. J Physiol 357:219–240
Diamond MR, Ross J, Morrone MC (2000) Extraretinal control of saccadic suppression. J Neurosci 20:3449–3455
Ditchburn RW (1955) Eye movements in relation to retinal action. Optica Acta 1:171–176
Feig S, Harting JK (1994) Ultrastructural studies of the primate lateral geniculate nucleus: morphology and spatial relationships of axon terminals arising from the retina, visual cortex (area 17), superior colliculus, parabigeminal nucleus, and pretectum of Galago crassicaudatus. J Comp Neurol 343:17–34
Fitzpatrick D, Diamond IT, Raczkowshi D (1989) Cholinergic and monoaminergic innervation of the cat’s thalamus: comparison of the lateral geniculate nucleus with other principal sensory nuclei. J Comp Neurol 288:647–675
Funk K, Eysel UT (1995) Pharmacological inactivation of pretectal nuclei reveals different modulatory effects on retino-geniculate transmission by X and Y cells. Vis Neurosci 12:21–33
Hanes D, Thompson K, Schall J (1995) Relationship of pressaccadic activity in frontal eye field and supplementary eye field to saccade initiation in macaque: Poisson spike train analysis. Exp Brain Res 103:85–96
Harting JK, van Lieshout DP, Hashikawa T, Weber JTl (1991) The parabigeminogeniculate projection: connectional studies in 8 mammals. J Comp Neurol 305:559
Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75(4):800–802
Ichida J, Royal D, Sary Gy, Xu X, Shostak Y, Schall J, Casagrande V (2001) Evidence for suppression of activity in both parvocellular (P) and magnocellular (M) lateral geniculate nucleus (LGN) cells during saccadic eye movements. Abstr SFN 2001
Ichida JM, Royal D, Sary Gy, Schall J, Casagrande V (2003) Are there significant onset latency differences between LGN cells that carry S cone signals compared to those that carry M or L cone signals? Abstr SFN 2003
Irvin GE, Casagrande VA, Norton TT (1993) Center/surround relationships of magnocellular, parvocellular, and koniocellular relay cells in primate lateral geniculate nucleus. Vis Neurosci 10(2):363–373
Jeannerod M, Putkonen PTS (1971) Lateral geniculate unit activity and eye movements: Saccade-locked changes in dark and in light. Exp Brain Res 13: 533–546
Johnson K, Balakrishnan (1994) Continuous univariate distributions, vols I and II, 2nd edn. John Wiley and Sons
Judge SJ, Richmond BJ, Chu FC (1980) Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20:535–538
Khayat PS, Spekreijse H, Roelfsema R (2004) Correlates of transsaccadic integration in primary visual cortex of the monkey. proceedings of the National Academy of Sciences 101:12712–12717
Kim H, Connors B (1993) Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J Neurosci 13:5301–5311
Latour PL (1962) Visual threshold during eye movements. Vision Res 2:261–262
Lee D, Malpeli JG (1998) Effects of saccades on the activity of neurons in the cat lateral geniculate nucleus. J Neurophysiol 79:922–936
Legendy CR, Salcman M (1985) Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol 53(4):926–939
Martin PR, White AJR, Goodchild AK, Wilder HD, Sefton AE (1997) Evidence that blue on cells are part of the third geniculocortical pathway in primates. Eur J Neurosci 9:1536–1541
Mason A, Nicoll A, Stratford K (1991) Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J Neurosci 11:72–84
McKinney B, Lewis E, Wills V, Richards JE (2002) Developmental changes in the main sequence using interesting visual stimuli. International Society for Infancy Studies, Toronto, CA
Mitrani L, Mateeff S, Yakimoff N (1970) Smearing of the retinal image during voluntary saccadic eye movements. Vision Res 10(5):405–409
Montero VM, Robles L (1971) Saccadic modulation of cell discharges in the lateral geniculate nucleus. Vis Res Suppl 3:253–268
Paxinos G, Huang X, Toga AW (2000) The rhesus monkey brain in stereotaxic coordinates. Academic Press
Ramcharan EJ, Gnadt JW, Sherman SM (2001) The effects of saccadic eye movements on the activity of geniculate relay neurons in the monkey. Vis Neurosci 18:253–258
Reppas JB, Usrey WM, Reid RC (2002) Saccadic eye movements modulate visual responses in the lateral geniculate nucleus. Neuron 35:961
Riggs LA, Merton PA, Morton HB (1974) Suppression of visual phosphenes during saccadic eye movements. Vision Res 14:997–1011
Ross J, Burr D, Morrone C (1996) Suppression of the magnocellular pathway during saccades. Behav Brain Res 80:1–8
Sayer RJ, Friedlander MJ, Redman SJ (1990) The time course and amplitude of EPSPs evoked at synapses between pairs of CA3/CA1 neurons in hippocampal slice. J Neurosci 10:826–836
Schmidt M (1996) Neurons in the cat pretectum that project to the dorsal lateral geniculate nucleus are activated during saccades. J Physiol 76:2907–2918
Schmidt M, Hoffman K-P (1966) Physiological characterization of pretectal neurons projecting to the lateral nucleus in the cat. Eur J Neurosci 4:318–326
Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG (1998) Signal timing across the macaque visual system. J Neurophysiol 79:3272–3278
Sherman SM, Koch C (1986) The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus. Exp Brain Res 63:1–20
Shioiri S, Cavanagh P (1989) Saccadic suppression of low-level motion. Vision Res 29:915–928
Starr A, Angel R, Yeates H (1969) Visual suppression during smooth following and saccadic eye movements. Vision Res 9(1):195–197
Thilo KV, Santoro L, Walsh V, Blakemore C (2004) The site of saccadic suppression. Nat Neurosci 7:13–14
Volkmann FC (1962) Vision during voluntary saccadic eye movements. J Opt Soc Am 52:571–578
Volkmann F (1986) Human visual suppression. Vision Res 26(9):1401–1416
White AJ, Solomon SG, Martin PR (2001) Spatial properties of koniocellular cells in the lateral geniculate nucleus of the marmoset Callithrix jacchus. J Physiol 533:519–535
Wiesel TN, Hubel DH (1966) Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol 29:1115–1116
Xu X, Ichida JM, Allison JD, Boyd JD, Bonds AB, Casagrande VA (2001) A comparison of koniocellular, magnocellular and parvocellular receptive field properties in the lateral geniculate nucleus of the owl monkey (Aotus trivirgatus). J Physiol 531:203–218
Xu XM, Bonds AB, Casagrande VA (2002) Modeling receptive-field structure of koniocellular, magnocellular, and parvocellular LGN cells in the owl monkey (Aotus trivirgatus). Vis Neurosci 19:703–711
Zhu JJ, Lo FS (1996) Time course of inhibition induced by a putative saccadic suppression circuit in the dorsal lateral geniculate nucleus of the rabbit. Brain Res Bull 41:281–291
Zuber BL, Stark L (1966) Saccadic suppression: elevation of visual threshold associated with saccadic eye movements. Exp Neurol 16:65–79
Zuber BL, Stark L, Lorber M (1966) Saccadic suppression of the pupillary light reflex. Exp Neurol 14:351–370
Acknowledgments
We are especially grateful to Mary Feurtado for her excellent assistance with animal care and anesthesia. We would also like to thank Jackie Munch and Kathryn Saclarides for help with experiments. Finally, we would like to thank Julia Mavity-Hudson, Yuliya Sokolova, Leanne Boucher, Veit Stuphorn, and Stephanie Shorter-Jacobi for their comments and suggestions on earlier drafts of this article. Supported by 1F31NS44691 (DWR) EY01778 (VAC), IBN-0234646 (VAC) EY08890 (JDS), and core grants EY08126 and HD15052.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Royal, D.W., Sáry, G., Schall, J.D. et al. Correlates of motor planning and postsaccadic fixation in the macaque monkey lateral geniculate nucleus. Exp Brain Res 168, 62–75 (2006). https://doi.org/10.1007/s00221-005-0093-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0093-z