Abstract
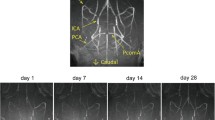
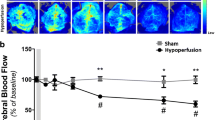
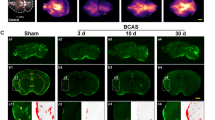
An animal model involving stepwise occlusion of the common carotid arteries (sCCAO) in DBA/2 mice is presented in which the right and left carotid arteries were permanently ligated within a time interval of four weeks. Thereafter, cerebral functional and structural parameters were determined at acute (15 min) and subchronic (1 day; 3, 7, and 14 days) time points after sCCAO. Quantitative changes in regional cerebral blood flow (rCBF) as determined by the [14C]iodoantipyrine method, energy state (ATP, phosphocreatine, ADP, AMP, adenosine) as shown by HPLC, brain histopathology, and neuronal densities were measured in both hemispheres. Acute sCCAO was accompanied by a drastic reduction in cerebral energy-rich phosphate concentrations, ATP and phosphocreatine, and in rCBF of more than 50%. In contrast, cortical adenosine increased around five-fold. Subchronic sCCAO, however, was associated with normalization in brain energy metabolites and near-complete restoration of rCBF, except in the caudate nucleus (−40%). No marked signs of necrotic or apoptotic cell destruction were detected. Thus, during the subchronic period, compensatory mechanisms are induced to counteract the drastic changes seen after acute vessel occlusion. In conclusion, this sCCAO mouse model may be useful for long-lasting investigations of stepwise deterioration contributing to chronic cerebrovascular disorders.


Similar content being viewed by others
References
Barone PC, Knudsen DJ, Nelson AH, Feuerstein GZ, Willette RN (1993) Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cerebr Blood F Met 13:683–692
Busch HJ, Buschmann IR, Mies G, Bode C, Hossmann KA (2003) Arteriogenesis in hypoperfused rat brain. J Cerebr Blood F Met 23:621–628
Coyle P, Panzenbeck MJ (1990) Collateral development after artery occlusion in Fisher 344 rats. Stroke 21:316–321
de la Torre JC (1997) Hemodynamic consequences of deformed microvessels in the brain in Alzheimer’s disease. Ann N Y Acad Sci 826:75–91
Eklöf B, Siesjö BK (1972) The effect of bilateral carotid artery ligation upon the blood flow and the energy state of the rat brain. Acta Physiol Scand 86:155–165
Erecinska, M, Silver, IA (1989) ATP and brain function. J Cereb Blood F Met 9:2–19
Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM (1994) Local cerebral blood flow during hibernation, a model of natural tolerance to ‘cerebral ischemia’. J Cereb Blood F Met 14:193–205
Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA (1997) Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57Black/6 mice. Stroke 28:1805–1811
Glazier SS, O’Rourke DM, Graham DI, Welsh FA (1994) Induction of ischemic tolerance following brief focal ischemia in rat brain. J Cereb Blood F Met 14:545–553
Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJM (2000) Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. Stroke 31:128–132
Izumi N, Yasuda H: A convenient cerebral ischemic model using mice (1983) J Cereb Blood F Met 3:S391–S392
Jay TM, Lucignani G, Crane AM, Jehle J, Sokoloff L (1988) Measurement of local cerebral blood flow with [14C]iodoantipyrine in the mouse. J Cereb Blood F Met 8:121–129
Kaliszewski C, Fernandez LA, Wicke JD (1988) Differences in mortality rate between abrupt and progressive carotid ligation in the gerbil: role of endogenous angiotensin II. J Cerebr Blood F Met 8:149–154
Kirsch JR, D’Alecy LG (1979) Effect of altered availability of energy-yielding substrates upon survival from hypoxia in mice. Stroke 10:288–291
Kirino T, Tsujita Y, Tamura A (1991) Induced tolerance to ischemia in gerbil hippocampal neurons. J Cerebr Blood F Met 11:299–307
Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T (1998) Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cerebr Blood F Met 18:570–579
MacMillan V, Judge D, Wisemann A, Settles D, Swain J, Davis J (1993) Mice expressing a bovine basic fibroblast growth factor transgene in the brain showed increased resistance to hypoxic-ischemic cerebral damage. Stroke 24:1735–1739
Murakami K, Kondo T, Kawase M, Chan PH (1998) The development of a new mouse model of global ischemia: focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res 780:304–310
Nakano S, Kato H, Kogure K (1989) Neuronal damage in the rat hippocampus in a new model of repeated reversible transient cerebral ischemia. Brain Res 490:178–180
Pappas BA, de la Torre JC, Davidson CM, Keyes MT, Fortin T (1996) Chronic reduction of cerebral blood flow in the adult rat: late-emerging CA1 cell loss and memory dysfunction. Brain Res 708:50–58
Phillis JW, Smith-Barbour M, O’Reagan MH, Perkins LM (1994) Amino acid and purine release in rat brain following temporary middle cerebral artery occlusion. Neurochem Res 19:1125–1130
Plaschke K (2004) Aspects of ageing in chronic cerebral oligaemia. Mechanisms of degeneration and compensation in rat models 2004. J Neural Transm (in press)
Plaschke K, Yun SW, Martin E, Hoyer S, Bardenheuer HJ (1999) Interrelation between cerebral energy metabolism and rat behaviour in a rat model of permanent brain vessel occlusion. Brain Res 830:320–329
Plaschke K, Weigand MA, Michel A, Martin E, Bardenheuer HJ (2000) Permanent cerebral hypoperfusion: ‘preconditioning-like’ effects on rat energy metabolism towards acute systemic hypotension. Brain Res 858:363–370
Plaschke K, Sommer C, Fahrner A, Amann K, Bardenheuer HJ, Knauth M (2003) Pronounced arterial collateralization was induced after permanent rat cerebral four-vessel occlusion. Relation to cerebral blood flow and neuropathology. J Neural Transm 110:719–32
Rebel A, Ulatowski JA, Joung K, Bucci E, Traystman RJ, Koehler RC (2002) Regional cerebral blood flow in cats with cross-linked hemoglobin transfusion during focal cerebral ischemia. Am J Physiol–Heart C 282:H832–H841
Sakurada O, Sokoloff L, Jacquet YF (1978) Local cerebral glucose utilization following injection of beta-endorphin into periaqueductal gray matter in the rat. Brain Res 153:403–407
Schaser KD, Settmacher U, Puhl G, Zhang L, Mittlmeier T, Stover JF, Vollmar B, Menger MD, Neuhaus P, Haas NP (2003) Noninvasive analysis of conjunctival microcirculation during carotid artery surgery reveals microvascular evidence of collateral compensation and stenosis-dependent adaptation. J Vasc Surg 37:789–797
Sheng H, Laskowitz DT, Pearlstein RD, Warner DS (1999) Characterization of a recovery global cerebral ischemia model in the mouse. J Neurosci Meth 88:103–109
Sommer C, Fahrner A, Kiessling M (2002) [3H] muscimol binding to gamma-aminobutyric acid (A) receptors is upregulated in CA1 neurons of the gerbil hippocampus in the ischemia-tolerant state. Stroke 33:1698–1705
Steen PA, Michenfelder JD (1978) Cerebral protection with barbiturates: relation to anesthetic effect. Stroke 9:140–142
Tachibana H, Meyer JS, Kitagawa Y, Tanahashi N, Kandula P, Rogers RL (1985) Xenon contrast CT-CBF measurements in parkinsonism and normal aging. J Am Geriatr Soc 33:413–421
Terashima T, Shobu S, Hoshimaru M, Uemura Y, Kikuchi H, Hashimoto N (1998) Consistent injury in the striatum of C57BL/6 mice after transient bilateral common carotid artery occlusion. Neurosurgery 43:900–907
Ueda H, Hashimoto T, Furuya E, Tagawa K, Kitagawa K, Matsumoto M, Yoneda S, Kimura K, Kamada T (1988) Changes in aerobic and anaerobic ATP-synthesizing activities in hypoxic mouse brain. J Biochem 104:81–86
Welsh FA, Sakamoto T, McKee AE, Sims RE (1987) Effect of lactacidosis on pyridine nucleotide stability during ischemia in mouse brain. J Neurochem 49:846–851
Wu C, Zhan RZ, Qi S, Fujihara H, Taga K, Shimoji K (2001) A forebrain ischemic preconditioning model established in C57Black/Crj6 mice. J Neurosci Meth 107:101–106
Yang G, Kitagawa K, Matsushita K, Mabuchi T, Yagita Y, Yanagihara T, Matsumoto M (1997) C57BL/6 strain is most susceptible to cerebral ischemia following bilateral common carotid occlusion among seven mouse strains: selective neuronal death in the murine transient forebrain ischemia. Brain Res 752:209–218
Acknowledgements
The authors would like to thank Roland Galmbacher, Stephan Hennes, Sigrid Kirchmeier, and Sebastian Hennig for their excellent technical assistance. This study was supported in part by the Medical Faculty of the University of Heidelberg (Forschungsschwerpunkt Geriatrie).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plaschke, K., Sommer, C., Schroeck, H. et al. A mouse model of cerebral oligemia: relation to brain histopathology, cerebral blood flow, and energy state. Exp Brain Res 162, 324–331 (2005). https://doi.org/10.1007/s00221-004-2177-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2177-6