Abstract
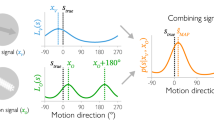
We compared quantitatively the psychometric capacity of human subjects to detect path-guided apparent motion (PAM) and the accuracy of cell ensembles in area 7a to code the same type of stimuli. Nine human subjects performed a detection task of PAM. They were instructed to indicate with a key-press whether they perceived a circularly moving object when five stimuli were flashed successively at the vertices of a regular pentagon. The stimuli were presented along a low contrast circular path with one of 33 speeds (150–600°/s). The average psychometric curve revealed that the threshold for PAM detection was 314°/s. The minimum and maximum thresholds for individual subjects were 277° and 378°/s, respectively. In addition, the activity of cells in area 7a that were modulated by the stimulus position in real or apparent motion was used in a multivariate linear regression analysis to recover the stimulus position over time. Real stimulus motion was decoded successfully from neural ensemble activity at all speeds. In contrast, the decoding of PAM was poor at low stimulus speeds but improved markedly above 300°/s: in fact, this was very close to the threshold above for human subjects to perceive continuous stimulus motion in this condition. These results suggest that the posterior parietal cortex is part of a high-level system that is directly involved in the dynamic representation of complex motion







Similar content being viewed by others
References
Andersen RA, Asanuma C, Essick G, Siegel RM (1990) Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol 296:65–113
Anstis SM (1980) The perception of apparent movement. Philos Trans R Soc Lond B Biol Sci 290:153–168
Battelli L, Cavanagh P, Intriligator J, Tramo MJ, Henaff MA, Michel F, Barton JJ (2001) Unilateral right parietal damage leads to bilateral deficit for high-level motion. Neuron 32:985–995
Britten KH, Shadlen MN, Newsome WT, Movshon JA (1992) The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12:4745–4765
Cavanagh P (1992) Attention-based motion perception. Science 257:1563–1565
Chatterjee SH, Freyd JJ, Shiffrar M (1996) Configural processing in the perception of apparent biological motion. J Exp Psychol Hum Percept Perform 22:916–929
Constantinidis C, Steinmetz MA (2001) Neuronal responses in area 7a to multiple-stimulus displays. I. neurons encode the location of the salient stimulus. Cereb Cortex 11:581–591
Dayan P, Abbott LF (2002) Theoretical neuroscience: computational and mathematical modeling of neural systems. MIT Press, Cambridge
De Valois R, Morgan HC (1974) Psychophysical studies of monkey vision. II. Squirrel monkey wavelength and saturation discrimination. Vision Res 14:69–73<1
De Valois RL, Morgan HC, Polson MC, Mead WR, Hull EM (1974) Psychophysical studies of monkey vision. I. Macaque luminosity and color vision tests. Vision Res 14:53–67
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47
Georgopoulos AP, Schwartz AB, Kettner RE (1986) Neuronal population coding of movement direction. Science 233:1416–1419
Georgopoulos AP, Lurito JT, Petrides M, Schwartz AB, Massey JT (1989) Mental rotation of the neuronal population vector. Science 243:234–236
Georgopoulos AP, Ashe J, Smyrnis N, Taira M (1992) Motor cortex and the coding of force. Science 256:1692–1695
Golomb B, Andersen RA, Nakayama K, MacLeod DI, Wong A (1985) Visual thresholds for shearing motion in monkey and man. Vision Res 25:813–820
Hernandez A, Zainos A, Romo R (2000) Neuronal correlates of sensory discrimination in the somatosensory cortex. Proc Natl Acad Sci U S A 97:6191–6196
Humphrey DR, Schmidt EM, Thompson WD (1970) Predicting measures of motor performance from multiple cortical spike trains. Science 170:758–762
Kolers PA (1972) Aspects of motion perception. Pergamon, New York
LaMotte RH, Mountcastle VB (1975) Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychological measurements. J Neurophysiol 38:539–559
Liu T, Slotnick SD, Yantis S (2004) Human MT+ mediates perceptual filling-in during apparent motion. Neuroimage 21:1772–1780
Lu ZL, Sperling G (2001) Three-systems theory of human visual motion perception: review and update. J Opt Soc Am A Opt Image Sci Vis 18:2331–2370
Merchant H, Zainos A, Hernandez A, Salinas E, Romo R (1997) Functional properties of primate putamen neurons during the categorization of tactile stimuli. J Neurophysiol 77:1132–1154
Merchant H, Battaglia-Mayer A, Georgopoulos AP (2001) Effects of optic flow in motor cortex and area 7a. J Neurophysiol 86:1937–1954
Merchant H, Battaglia-Mayer A, Georgopoulos AP (2003a) Interception of real and apparent motion targets: psychophysics in humans and monkeys. Exp Brain Res 152:106–112
Merchant H, Battaglia-Mayer A, Georgopoulos AP (2003b) Functional organization of parietal neuronal responses to optic flow stimuli. J Neurophysiol 90:675–682
Merchant H, Battaglia-Mayer A, Georgopoulos AP (2004a) Neural responses in motor cortex and area 7a to real and apparent motion. Exp Brain Res 154:291–307
Merchant H, Battaglia-Mayer A, Georgopoulos AP (2004b) Neural responses during interception of real and apparent circularly moving targets in Motor Cortex and Area 7a. Cereb Cortex 14:314–331
Mikami A (1991) Direction selective neurons respond to short-range and long-range apparent motion stimuli in macaque visual area MT. Int J Neurosci 61:101–112
Motter BC, Mountcastle VB (1981) The functional properties of the light-sensitive neurons of the posterior parietal cortex studied in waking monkeys: foveal sparing and opponent vector organization. J Neurosci 1:3–26
Mountcastle VB, LaMotte RH, Carli G (1972) Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. J Neurophysiol 35:122–136
Muckli L, Kriegeskorte N, Lanfermann H, Zanella FE, Singer W, Goebel R (2002) Apparent motion: event-related functional magnetic resonance imaging of perceptual switches and states J Neurosci 22:RC219
Newsome WT, Mikami A, Wurtz RH (1986) Motion selectivity in macaque visual cortex III Psychophysics and physiology of apparent motion. J Neurophysiol 55:1340–1351
Parker AJ, Newsome WT (1998) Sense and the single neuron: probing the physiology of perception. Annu Rev Neurosci 21:227–277
Pouget A, Dayan P, Zemel RS (2003) Inference and computation with population codes. Annu Rev Neurosci 26:381–410
Ramachandran VS, Anstis SM (1983) Perceptual organization in moving patterns. Nature 304:529–531
Seiffert AE, Cavanagh P (1998) Position displacement, not velocity, is the cue to motion detection of second-order stimuli Vision Res 38:3569–3582
Shepard RN (1984) Ecological constraints on internal representation: resonant kinematics of perceiving, imagining, thinking, and dreaming. Psychol Rev 91:417–447
Shepard RN, Zare SL (1983) Path-guided apparent motion. Science 220:632–634
Siegel RM, Read HL (1997) Analysis of optic flow in the monkey parietal area 7a. Cereb Cortex 7:327–346
Sterzer P, Russ MO, Preibisch C, Kleinschmidt A (2002) Neural correlates of spontaneous direction reversals in ambiguous apparent visual motion. Neuroimage 15:908–916
Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB (1968) The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31:301–334
Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, Kim J, Biggs SJ, Srinivasan MA, Nicolelis MA (2000) Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature 408:361–365
Williams ZM, Elfar JC, Eskandar EN, Toth LJ, Assad JA (2003) Parietal activity and the perceived direction of ambiguous apparent motion. Nat Neurosci 6:616–623
Yantis S, Nakama T (1998) Visual interactions in the path of apparent motion. Nat Neurosci 1:508–512
Acknowledgements
This work was supported by United States Public Health Service grant PSMH48185, the United States Department of Veterans Affairs, and the American Legion Brain Sciences Chair.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Merchant, H., Battaglia-Mayer, A. & Georgopoulos, A.P. Decoding of path-guided apparent motion from neural ensembles in posterior parietal cortex. Exp Brain Res 161, 532–540 (2005). https://doi.org/10.1007/s00221-004-2100-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2100-1