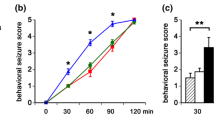
Abstract
Prolonged seizures induced by neurotoxins or intracranial electrical stimulation provoke death of hippocampal neurons, which results in conspicuous learning and memory deficits. We examined whether repeated brief seizures elicited by electroconvulsive shock (ECS) can also deteriorate hippocampal structure and function. Adult Wistar rats were administered six ECS seizures, the first five of which were 24 h apart, whilst the last two were spaced by a 2-h interval. Following a 2-month recovery period, the cognitive status of the animals was assessed using the water maze task. ECS-treated animals were incapable of learning the constant platform position version of this task during the first 4 days of training, but performed similarly to control rats throughout the rest of the acquisition period, on the probe trial, and on the variable platform position and visible platform tasks. The results of the morphological analysis showed that the total number of hippocampal pyramidal neurons and dentate gyrus granule cells were similar in control and ECS-treated rats. However, ECS treatment caused loss of approximately 17% of cells in the hilus of the dentate gyrus, which was accompanied by significant mossy fiber sprouting into the dentate inner molecular layer. In addition, we found that the ECS-induced decrease in the total number of hilar cells was not due to loss of inhibitory interneurons immunoreactive to somatostatin. These findings support the view that ECS-induced seizures can produce a number of morphological and functional changes in the rat hippocampal formation, which qualitatively resemble those previously described in other seizure models.




Similar content being viewed by others
References
Adams B, Sazgar M, Osehobo P, Van der Zee CE, Diamond J, Fahnestock M, Racine RJ (1997) Nerve growth factor accelerates seizure development, enhances mossy fiber sprouting, and attenuates seizure-induced decreases in neuronal density in the kindling model of epilepsy. J Neurosci 17:5288–5296
Amaral DG, Witter MP (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31:571–591
Armstrong DD (1993) The neuropathology of temporal lobe epilepsy. J Neuropathol Exp Neurol 52:433–443
Ben-Ari Y, Tremblay E, Ottersen OP, Meldrum BS (1980) The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res 191:79–97
Bengzon J, Kokaia Z, Elmér E, Nanobashvili A, Kokaia M, Lindvall O (1997) Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A 94:10432–10437
Bertram EH III, Lothman EW (1993) Morphometric effects of intermittent kindled seizures and limbic status epilepticus in the dentate gyrus of the rat. Brain Res 603:25–31
Binder DK, McNamara JO (1998) Kindling: a pathologic activity-drive structural and functional plasticity in mature brain. In: Corcoran ME, Moshe SL (eds) Kindling, vol 5. Plenum, New York, pp 245–254
Bouilleret V, Loup F, Kiener T, Marescaux C, Fritschy JM (2000) Early loss of interneurons and delayed subunit-specific changes in GABA(A)-receptor expression in a mouse model of mesial temporal lobe epilepsy. Hippocampus 10:305–324
Bragin A, Jandó G, Nádasdy Z, van Landeghem M, Buzsáki G (1995) Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J Neurophysiol 73:1691–1705
Buckmaster PS, Dudek FE (1997) Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol 385:385–404
Buckmaster PS, Jongen-Rêlo AL (1999) Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci 19:9519–9529
Cavazos JE, Sutula TP (1990) Progressive neuronal loss induced by kindling: a possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Res 527:1–6
Cavazos JE, Golarai G, Sutula TP (1991) Mossy fiber synaptic reorganization induced by kindling: time course of development, progression and permanence. J Neurosci 11:2795–2803
Cavazos JE, Das I, Sutula TP (1994) Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci 14:3106–3121
Dalby NO, Tønder N, Wolby DP, West M, Finsen B, Bolwig TG (1996) No loss of hippocampal hilar somatostatinergic neurons after repeated electroconvulsive shock: a combined stereological and in situ hybridization study. Biol Psychiatry 40:54–60
Danscher G, Zimmer J (1978) An improved Timm sulphide silver method for light and electron microscopic localization of heavy metals in biological tissues. Histochemistry 55:27–40
Deller T, Katona I, Cozzari C, Frotscher M, Freund TF (1999) Cholinergic innervation of mossy cells in the rat fascia dentata. Hippocampus 9:314–320
Devanand DP, Dwork AJ, Hutchinson ER, Bolwig TG, Sackeim HA (1994) Does ECT alter brain structure? Am J Psychiatry 151:957–970
Fisher RS (1989) Animal models of the epilepsies. Brain Res Rev 14:245–278
Freund TF, Ylinen A, Miettinen R, Pitkänen A, Lahtinen H, Baimbridge KG, Riekkinen PJ (1992) Pattern of neuronal death in the rat hippocampus after status epilepticus. Relationship to calcium binding protein content and ischemic vulnerability. Brain Res Bull 28:27–38
Frotscher M, Seress L, Schwerdtfeger WK, Buhl E (1991) The mossy cells of the fascia dentata: a comparative study of their fine structure and synaptic connections in rodents and primates. J Comp Neurol 312:145–163
Gilbert TH, McNamara RK, Corcoran ME (1996) Kindling of hippocampal field CA1 impairs spatial learning and retention in the Morris water maze. Behav Brain Res 82:57–66
Gombos Z, Spiller A, Cottrell GA, Racine RJ, Burnham WM (1999) Mossy fiber sprouting induced by repeated electroconvulsive shock seizures. Brain Res 844:28–33
Gundersen HJG, Jensen EB (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147:229–263
Gundersen HJG, Bendsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ (1988) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand 96:379–394
Gundersen HJG, Jensen EBV, Kiêu K, Nielsen J (1999) The efficiency of systematic sampling in stereology—reconsidered. J Microsc 193:199–211
Hannesson DK, Howland J, Pollock M, Mohapel P, Wallace AE, Corcoran ME (2001) Dorsal hippocampal kindling produces a selective and enduring disruption of hippocampally mediated behavior. J Neurosci 21:4443–4450
Hauser WA (1983) Status epilepticus: frequency, etiology, and neurological sequelae. Adv Neurol 34:3–14
He Y, Janssen WGM, Vissavajjhala P, Morrison JH (1998) Synaptic distribution of GluR2 in hippocampal GABAergic interneurons and pyramidal cells: a double-label immunogold analysis. Exp Neurol 150:1–13
Houser CR, Esclapez M (1996) Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res 26:207–218
Hsieh PF (1999) Neuropathology of limbic status epilepticus induced by electrical stimulation of naive rats. Neurol Res 21:399–403
Kelsey JE, Sanderson KL, Frye CA (2000) Perforant path stimulation in rats produces seizures, loss of hippocampal neurons, and a deficit in spatial mapping which are reduced by prior MK-801. Behav Brain Res 107:59–69
Lemos T, Cavalheiro EA (1995) Suppression of pilocarpine-induced status epilepticus and the late development of epilepsy in rats. Exp Brain Res 102:423–428
Ling EA, Paterson JA, Privat A, Mori S, Leblond CP (1973) Investigation of glial cells in semithin sections. I. Identification of glial cells in the brain of young rats. J Comp Neurol 149:43–72
Lopes da Silva FH, Gorter JA, Wadman WJ (1986) Kindling of the hippocampus induces spatial memory deficits in the rat. Neurosci Lett 63:115–120
McNamara JO (1999) Emerging insights into the genesis of epilepsy. Nature 399:A15–A22
Meldrum BS (1993) Excitotoxicity and selective neuronal loss in epilepsy. Brain Pathol 3:405–412
Mizumori SJ, Ragozzino KE, Cooper BG, Leutgeb S (1999) Hippocampal representational organization and spatial context. Hippocampus 9:444–451
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Olton DS (1983) Memory functions and the hippocampus. In: Seifert W (ed) Neurobiology of the hippocampus. Academic Press, New York, pp 335–374
Paulsen O, Moser EI (1998) A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci 21:273–278
Regeur L, Pakkenberg B (1989) Optimizing sampling designs for volume measurements of components of human brain using a stereological method. J Microsc 155:113–121
Ribak CE, Seress L, Amaral DG (1985) The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol 14:835–857
Rowley HL, Marsden CA, Martin KF (1997) Generalised seizure-induced changes in rat hippocampal glutamate but not GABA release are potentiated by repeated seizures. Neurosci Lett 234:143–146
Salmenperä T, Kälviäinen R, Partanen K, Pitkänen A (1998) Hippocampal damage caused by seizures in temporal lobe epilepsy. Lancet 351:35
Schwarzer C, Sperk G, Samanin R, Rizzi M, Gariboldi M, Vezzani A (1996) Neuropeptides—immunoreactivity and their mRNA expression in kindling: functional implications for limbic epileptogenesis. Brain Res Rev 22:27–50
Scott BW, Wojtowicz JM, Burnham WM (2000) Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol 165:231–236
Sloviter RS (1987) Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science 235:73–76
Sloviter RS (1991) Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus 1:41–66
Sloviter RS, Dean E, Sollas AL, Goodman JH (1996) Apoptosis and necrosis induced in different hippocampal neuron populations by repetitive perforant path stimulation in the rat. J Comp Neurol 366:516–533
Sloviter RS, Ali-Akbarian L, Horvath KD, Menkens KA (2001) Substance P receptor expression by inhibitory interneurons of the rat hippocampus: enhanced detection using improved immunocytochemical methods for the preservation and colocalization of GABA and other neuronal markers. J Comp Neurol 430:283–305
Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M (2003) “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol 459:44–76
Sperk G (1994) Kainic acid seizures in the rat. Prog Neurobiol 42:1–32
Sutula TP, Cavazos JE, Woodard AR (1994) Long-term structural and functional alterations induced in the hippocampus by kindling: implications for memory dysfunction and the development of epilepsy. Hippocampus 4:254–258
Sutula T, Lauersdorf S, Lynch M, Jurgella C, Woodard A (1995) Deficits in radial arm maze performance in kindled rats: evidence for long-lasting memory dysfunction induced by repeated brief seizures. J Neurosci 15:8295–8301
Tauck DL, Nadler JV (1985) Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci 5:1016–1022
Turski WA, Cavalheiro EA, Ikonomidou C, Mello LE, Bortolotto ZA, Turski L (1985) Effects of aminophylline and 2-chloroadenosine on seizures produced by pilocarpine in rats: morphological and electroencephalographic correlates. Brain Res 361:309–323
Tuunanen J, Pitkänen A (2000) Do seizures cause neuronal damage in rat amygdala kindling? Epilepsy Res 39:171–176
Vaidya VA, Siuciak JA, Du F, Duman RS (1999) Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience 89:157–166
Vida I, Frotscher M (2000) A hippocampal interneuron associated with the mossy fiber system. Proc Natl Acad Sci U S A 97:1275–1280
Vogt KE, Nicoll RA (1999) Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci U S A 96:1118–1122
West MJ, Slomianka L, Gundersen HJG (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497
Ylinen A, Lahtinen H, Sirviö J, Partanen J, Asikainen A, Gulyas A, Freund TF, Riekkinen P (1991) Behavioural, electrophysiological and histopathological changes following sustained stimulation of the perforant pathway input to the hippocampus: effect of the NMDA receptor antagonist, CGP 39551. Brain Res 553:195–200
Zhang LX, Smith MA, Li XL, Weiss SR, Post RM (1998) Apoptosis of hippocampal neurons after amygdala kindled seizures. Mol Brain Res 55:198–208
Acknowledgements
This work was supported by Fundação para a Ciência e a Tecnologia, Unit 121/94, Grant SFRH/BPD/1583/2000. The authors would like to thank Dr. J.P. Andrade and Dr. R.M. Mesquita for their assistance with the immunocytochemistry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lukoyanov, N.V., Sá, M.J., Madeira, M.D. et al. Selective loss of hilar neurons and impairment of initial learning in rats after repeated administration of electroconvulsive shock seizures. Exp Brain Res 154, 192–200 (2004). https://doi.org/10.1007/s00221-003-1658-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1658-3