Abstract
Vital wheat gluten plays an important role in the food industry, especially in baking to help standardize dough properties and improve bread volume. However, a fundamental characterization of a wide variety of vital gluten samples is not available so far. This would be necessary to relate compositional characteristics to the production process. Therefore, we analyzed the content of crude protein, starch, lipids and ash, oil and water absorption capacity, particle size distribution, gluten protein composition and spectroscopic properties of 39 vital gluten samples from 6 different suppliers. Principle component analysis of all analytical parameters revealed that the samples from one specialized vital gluten manufacturer had a different composition and a greater variability compared to all other samples from wheat starch producers. While the composition of vital gluten samples from the same manufacturer was similar and the score plot showed a cluster formation for samples from three suppliers, the variability over all samples was comparatively low. The samples from the other suppliers were too similar altogether so that it was hardly possible to identify clear differences, also related to functionality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vital wheat gluten is defined in the Codex Standard 163–1987 [1] as a wheat protein product with a high viscoelasticity after hydration. The dried powder has to fulfill the following requirements to be called vital gluten: a crude protein content of ≥ 80% (dry matter basis, N × 6.25), a moisture content of ≤ 10%, an ash content of ≤ 2%, and a crude fiber content of ≤ 1.5%. Gluten is isolated from wheat flour by washing out non-protein constituents such as starch or soluble carbohydrates with water. This simple process for producing gluten was first described in 1728 by an Italian chemist named Beccari [2]. Today’s extraction methods such as the Martin process or the batter process are still based on this principle [3]. The mild drying conditions of wet gluten ensure that its functional properties, e.g., cohesivity, elasticity and viscosity, are largely preserved and become effective again after rehydration [4]. Due to these functional properties, vital gluten is becoming increasingly important for the food, feed, and non-food industries, especially the baking industry. The use of vital gluten leads to protein enrichment in low-protein flours, to an improved technological quality (dough firmness, mixing tolerance and handling of the dough) and an increase in gas-holding capacity that finally results in higher bread volumes [5]. The viscoelastic properties of vital gluten are mainly determined by the interaction between gliadins and glutenins, the storage proteins in wheat. Gliadins are predominantly monomeric and can be further divided into ω5-, ω1,2-, α- and γ-gliadins according to homologies in their relative molecular masses (Mr) and amino acid sequences [6, 7]. The fact that they form intramolecular disulfide bonds make them soluble in aqueous alcohol, e.g., 60% ethanol. In contrast, glutenins are polymeric structures interconnected by disulfide bonds and are, therefore, not soluble in aqueous alcohol unless they are reduced at temperatures above 60 °C with a reducing agent such as dithiothreitol. The Mr allows a further division into high-molecular-weight (HMW, Mr: 70,000–90,000) and low-molecular-weight (LMW, Mr: 30,000–45,000) glutenin subunits (GS) [7]. Due to their ability to form intermolecular disulfide bonds, glutenins can cross-link and form a gluten network, resulting in increased dough elasticity and gluten strength. The presence of gliadins weakens the gluten network through their incorporation into the network structure and leads to a higher viscosity [8]. A good balance between gliadins and glutenins is desired to achieve a good baking performance. Besides the gliadin/glutenin ratio, the manufacturing process itself has an influence on the quality of vital gluten. During manufacturing, wet gluten has to be dried to extend its shelf life. Weegels et al. [4] described the drying procedure as the most critical step regarding the devitalizing effect of heat on gluten. In the industrial production of gluten, ‘ring’ or ‘flash’ drying is used. The principle involves reducing the moisture by adding wet gluten to dried gluten and simultaneous drying using heat [9]. The exact temperature cannot be determined during production as it depends on the quantity of gluten in the dryer. This temperature fluctuation can lead to differences in the quality of vital gluten due to heat sensitivity. Up to now, there are no in-depth studies that have examined a sufficiently large number of vital gluten samples to study structure–function relationships. Recently, baking experiments using 2 different recipes allowed a classification of 39 vital gluten samples into different quality classes according to their specific volume. However, the correlations of functionality to different parameters describing gluten protein composition were either weak or non-existent [10]. Based on the hypothesis that the composition and the structure of the major and minor components have an influence on the functionality of vital gluten, the aim of this study was to fundamentally characterize these vital gluten samples regarding protein, ash, lipid and starch content, particle size distribution, oil and water absorption capacity as well as gluten protein composition and Mr distribution. All parameters were combined in a principle component analysis (PCA) to find out whether vital gluten quality is manufacturer dependent. An additional aim was to analyze the secondary protein structure of these vital gluten samples by circular dichroism (CD) spectroscopy and near-infrared spectroscopy (NIRS).
Materials and methods
Materials
Vital wheat gluten samples (G1–G39) were provided by six suppliers, four of them manufacturers, the other two distributors. Vital gluten G1–G18, G19–G22, G23–G28, G29–G33, G34–G35, and G36–G39 came each from the same supplier. G1–G18 were from one of the distributors and were further subcategorized into four groups (G1, G2–G6, G7–G11, and G12–G18) based on the information of the supplier. G2–G6 can be assigned to the manufacturer of G34–G35, while the other vital gluten samples came from three unknown manufacturers. One manufacturer specializes in the production of vital gluten (G19–G22), the other manufacturers obtain vital gluten as a co-product of wheat starch production. Details about the production process were not disclosed by the suppliers due to confidentiality obligations, so that the effect of various production parameters on the structural characteristics could not be evaluated. All reagents were purchased from Merck KGaA (Darmstadt, Germany) or Sigma-Aldrich (Steinheim, Germany) at analytical or higher grade.
Determination of the basic composition
The moisture content of vital gluten samples was determined using the infrared moisture analyzer MA35 (Sartorius AG, Goettingen, Germany). Vital gluten (3.5 g) was heated up to 100 °C until the residual weight remained constant. The instrument automatically calculated the moisture content as percentile weight loss in relation to the original weight. The ash content was measured according to ICC standard method 104/1. Vital gluten (5 ± 0.1 g) was heated at 900 °C for 3 h. The weight before and after heating and the moisture content of the sample were used to calculate the ash content. The amount of total starch in the vital gluten samples was analyzed photometrically with the total starch enzyme kit (Megazyme International Ireland Ltd., Wicklow, Ireland). The fat content of the samples was determined with the Soxhlet method using 2.5 ± 0.05 g sample and petroleum ether as a solvent. The samples remained in the Soxhlet apparatus at 80 °C for 3 h. After evaporating the solvent with a rotary evaporator and drying the flasks overnight, the remaining lipids were measured gravimetrically. The TruSpec Nitrogen Analyzer (Leco, Kirchheim, Germany) was utilized to analyze the nitrogen content according to the method of Dumas. The crude protein content was calculated from the nitrogen content using a conversion factor of 6.25, as stated in Codex Standard 163–1987 [1]. All measurements were performed in triplicates.
Oil and water absorption capacity of vital gluten
The oil and water absorption capacity was analyzed in triplicates according to the method of Kaushik et al. [11]. Therefore, 500 mg vital gluten were mixed with 10 ml pure soy oil (Kunella Feinkost GmbH, Cottbus, Germany) or distilled water, respectively, and shaken for 1 h at 900 × g. After centrifugation at 2000 × g (22 °C, 30 min), the weight of the pellet was determined and used to calculate the absorption capacity (AC) with the following modified formula:
Particle size distribution
The particle size distribution was measured by static light scattering with a Mastersizer 3000 instrument using the Aero S unit for dry powders (Malvern Instruments Ltd, Worcestershire, UK) as described in Jakobi et al. [12]. The particle size of the dry dispersion was determined according to the Mie theory with a refractive index of 1. For each measurement, the average of 10 spectra was calculated. A range of obscuration of 1–8% was used for the measurement. The span is calculated as (D90 − D10)/D50.
Determination of the gluten protein composition by RP-HPLC
Vital gluten (20 mg) was extracted first with 60% (v/v) aqueous ethanol (3 × 1.5 ml) for 10 min at 22 °C (gliadins) and then with 50% (v/v) propan-1-ol, 0.05 mol/l Tris–HCl (pH 7.5), 2 mol/l (w/v) urea and 1% (w/v) dithiothreitol (DTT) (3 × 1.5 ml, 60 °C and under nitrogen atmosphere) for 30 min (glutenins). After centrifugation for 25 min at 4600×g and 22 °C, appropriate extracts were combined and diluted to 5.0 ml with the respective solvents. Protein fractions were quantitated by reversed-phase (RP)-HPLC analysis as described in Schopf and Scherf [13]. The calibration and the calculation of protein contents was established using PWG–gliadin (11.6–46.6 µg, dissolved in 60% (v/v) ethanol) [14].
Determination of extractable and non-extractable proteins by GP-HPLC
The extractable and non-extractable protein content was determined according to the method of Batey et al. [15]. To obtain the extractable protein fraction, 15 mg of vital gluten were extracted with 5 ml extraction solution (acetonitrile/water (1:1, v/v), 0.1% trifluoroacetic acid (TFA) (v/v)) by shaking for 30 min at 900×g and 22 °C, followed by centrifugation for 20 min at 4500×g and 22 °C. The residue was then extracted a second time essentially in the same way, except for an additional sonication step for 40 s at the beginning of the procedure to get the non-extractable protein fraction. Both extracts were analyzed by gel permeation (GP)-HPLC using a Hitachi Merck instrument (VWR) with a BioSep-SEC-s4000 column (300 × 4.6 mm, Phenomenex) under isocratic conditions with acetonitrile/water (1:1, v/v) containing 0.1% TFA (v/v) at a flow rate of 0.3 ml/min and 22 °C. The injection volume was 20 µl. UV detection was carried out at 210 nm. PWG–gliadin (2.5 mg/ml) was used as standard material [14] to calibrate the HPLC-system and finally to calculate the protein concentration of extractable and non-extractable proteins. The obtained peaks had a retention time of 6.0 -13.0 min. For both fractions, a further division into high-molecular-weight (HMW) and low-molecular-weight (LMW) was made, resulting in peaks from 6.0–9.2 min for HMW and 9.2–13.0 min for LMW.
Determination of SDS-soluble proteins and glutenin macropolymer by GP-HPLC
For the determination of the content of SDS-soluble (SDSS) proteins, 20 mg of vital gluten were extracted with 1% (w/v) SDS and 0.05 mol/l NaH2PO4 (pH 6.9) (2 × 1.0 ml). The extraction procedure involved vortex mixing for 2 min, then magnetic stirring for 30 min at 22 °C, followed by centrifugation for 25 min at 4600×g and 22 °C. To obtain the glutenin macropolymer (GMP), the residue was then extracted using the same procedure with 50% (v/v) 1-propanol, 0.05 mol/l Na2HPO4/KH2PO4 (pH 7.5) and 1% (w/v) DTT (2 × 1.0 ml, 60 °C, under nitrogen atmosphere) [16]. Both extracts were analyzed by gel permeation (GP)-HPLC using a Hitachi Merck instrument (VWR, Darmstadt, Germany) with a BioSep-SEC-s3000 column (300 × 4.6 mm, Phenomenex, Aschaffenburg, Germany) under isocratic conditions with acetonitrile/water (1:1, v/v) containing 0.1% TFA (v/v) at a flow rate of 0.3 ml/min and 22 °C. The injection volume was 20 µl. UV detection was carried out at 210 nm. PWG–gliadin (2.5 mg/ml) was used to calibrate the HPLC system [14].
Near-infrared spectroscopy
Near-infrared (NIR) spectra (12,800–4000 cm−1) of vital gluten samples were recorded using the Tango FT-IR spectrometer (Bruker Optik GmbH, Ettlingen, Germany) with the software OPUS 7.8. An average of 64 scans per spectrum was calculated with a resolution of 8 cm−1. The background spectra were generated with a clean empty cell. For each spectrum, the absolute and mathematical area was calculated every 50 cm−1 in the range from 7000 to 4000 cm−1.
Circular dichroism spectroscopy
Circular dichroism (CD) spectra (200–280 nm) were measured using the Chirascan Plus CD spectrometer (Applied Photophysics Ltd., Leatherhead, UK). The temperature was held constant at 20 °C during the measurement. An average of 10 scans per spectrum was made. Gliadins, glutenins and gluten of each vital gluten sample (G1–G39) were analyzed. The gliadins were obtained by extraction with 60% aqueous ethanol as described. For the glutenins and gluten, the sample preparation was made in the same manner as for the non-extractable protein fraction according to Batey et al. [15]. The background spectra were recorded with the appropriate solvents before each measurement.
Statistical analysis
Origin® 2019 (OriginLab Corporation, Northampton, USA) was used to collect and evaluate the data. Principal component analysis (PCA) was applied individually for each analysis, but also for a combination of all analytical results to check for variation and correlation between the vital gluten samples. One-way analysis of variance (ANOVA) with Tukey’s test (p ≤ 0.05) was performed by SigmaPlot 12.0 (Systat Software, San José, CA, USA).
Results and discussion
Basic composition of vital gluten
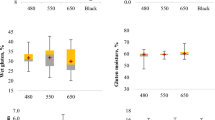
The basic composition of the vital gluten samples G1–G39 is summarized in Table 1. The moisture content was between 4.8 (G1) and 7.4% (G19), the ash content varied from 0.7 (G20) to 1.2% (G1) and the crude protein content ranged from 81.6 (G13) to 95.7% (G20). These values were consistent with the definition of vital gluten in the Codex Standard 163–1987 [1]. The starch content of the vital gluten samples G1–G39 was between 2.7 (G22) and 7.9% (G27). During the production of vital gluten, starch is separated from wheat flour by washing with water. Depending on the process, residual starch remains in the end product and starch contents from 7.1 to 15.5% are common [17]. The lipid content of the vital gluten samples G1–G39 ranged from 0.6 (G31) to 2.1% (G18). In the literature, similar lipid contents of 0.8–2.7% can be found [17, 18].
Oil and water absorption capacity
The oil absorption capacity (OAC) of the vital gluten samples G1–G39 varied from 98.5 (G21) to 129.1% (G14) (Table 1). Overall, the OAC of most vital gluten samples was similar. There were only some significant differences, e.g., G21 with the lowest OAC differed significantly from the 7 vital gluten samples with the highest OAC, while similar OACs were identified for the remaining 31 vital gluten samples. In this study, the OAC was calculated considering the sample weight, resulting in generally lower values compared to Kaushik et al., who reported 246.2–356.0% [11]. They calculated the OAC and WAC by dividing the weight of sediment by the weight of sample and multiplying the result by 100. The water absorption capacity (WAC) of the vital gluten samples G1–G39 ranged from 117.4 (G18) to 190.3% (G14) (Table 1). Interestingly, similar WACs were observed for several vital gluten samples from the same manufacturer. For example, G19–G22, G23–G28, as well as G3–G6 and G34–G35 showed no significant differences in their WAC. WACs of 249.9–354.2% for dried wheat gluten were reported by Kaushik et al. [11]. These values were higher compared to those found in this study, but as already mentioned for the calculation of the OAC, the sample weight was also considered in our study, which led to lower values. Especially, the WAC has been shown to be an important parameter determining the functionality of vital gluten when used in baking experiments, because it influences gluten network formation and the interaction of water molecules with both gluten polymers and starch [19].
Particle size distribution
The particle size distribution provides information about the diameter of the particles by three different key parameters: D10, D50, and D90. D50 represents the median diameter where 50% of the total particle volume is generated by particles with a diameter smaller than D50. The diameter where 90% are smaller and 10% are larger is called D90 and D10 defines the diameter where 10% are smaller and 90% larger. Vital gluten samples G1–G39 had values from 5.8 (G38) to 34.5 µm (G19) for D10, from 33.6 (G38) to 111.0 µm (G21) for D50 and from 93.2 (G28) to 302.0 µm (G21) for D90 (Table 2). The values for the width of the distribution (span) varied between 1.8 (G20) and 3.5 (G33). Overall, vital gluten samples G1–G39 showed similar particle size distributions except for G19–G21. Since we had no further information on the production process for each sample, it was not possible to link the particle size distribution to potential functionality. Considering the overall similarity of the D10, D50, and D90 values, we concluded that the particle size distribution did not appear to have a large influence on the characteristics of the samples G1–G39. This is in line with Wadhawan and Bushuk [20], who analyzed 27 commercial gluten samples and reported that the particle size had no significant effect on water absorption and functionality.
Gluten protein composition
A combination of modified Osborne fractionation and RP-HPLC [21] was applied to quantitate ω5-, ωb-, ω1,2-, α- and γ–gliadins, as well as HMW–GS and LMW–GS in the vital gluten samples G1–G39 (Table 3). G27 had the lowest gluten content with 687.8 mg/g, while G20 had the highest content with 944.0 mg/g. The recovery rate of the gluten content using RP-HPLC compared to the corresponding crude protein content was 85.9% or higher. Overall, G1–G39 showed similarities in their gliadin and glutenin distribution. Most variations were observed for the ω5–gliadins with a coefficient of variation (CV) of 0.15 and values ranging from 13.8 (G30) to 27.5 mg/g (G20). For the glutenins, the highest CV (0.11) was found for HMW–GS. G14 had the smallest content with 63.2 mg/g, whereas G36 had the highest content with 100.1 mg/g. The gliadin/glutenin ratio was between 1.5 (G36) and 2.7 (G15 and G18). Goesaert et al. [22] showed that a balanced gliadin/glutenin ratio is necessary to achieve good bread making quality in wheat flour. However, recent investigations showed that the gliadin/glutenin ratio was not sufficiently reliable to predict the baking performance of vital gluten samples [10]. Vital gluten samples from one manufacturer showed predominantly higher similarities in their protein distribution compared to samples from other manufacturers.
Extractable and non-extractable proteins
The extractable (EP) and non-extractable proteins (NEP) were isolated according to Batey et al. [15] and analyzed by GP-HPLC (Table 4). Values from 186.2 (G23) to 321.1 mg/g (G1) were observed for HMW-EP and 406.5 mg/g (G16) to 552.3 mg/g (G20) for LMW-EP. The NEP ranged between 12.1 (G6) and 41.6 mg/g (G7) for HMW-NEP and 16.6 mg/g (G6) and 24.6 mg/g (G38) for LMW-NEP. In total, the protein contents were between 637.1 (G23) and 887.0 mg/g (G20). In most cases, the vital gluten samples from one manufacturer had similar protein distributions.
SDSS proteins and GMP
The SDSS proteins and the GMP were extracted by the method of Gupta et al. [16] (Table 4). The total protein was between 530.8 (G22) and 787.2 mg/g (G27) and the SDSS proteins were between 449.4 (G22) and 718.7 mg/g (G26). For the GMP, the absolute protein content was 7.7 mg/g (G26) to 27.1 mg/g (G21) for LMW–GMP and from 25.6 (G26) to 85.7 mg/g (G20) for HMW–GMP, resulting in a total GMP content of 33.3 mg/g (G26) to 83.6 mg/g (G21). Vital gluten samples G19–G22 had higher GMP contents compared to the other vital gluten samples. In general, a high GMP content is associated with good baking performance in wheat flour [23], but our results indicated no correlation (p = 0.49) between the GMP content of vital gluten and the specific volumes of two independent baking experiments reported in Schopf and Scherf [10].
Comparison of GP-HPLC methods
Both GP-HPLC methods are based on the principle of gel permeation chromatography and provide information on the solubility in different extraction solutions, the Mr distribution and the ratio of soluble to insoluble proteins of the vital gluten samples. However, different pore sizes in the column, BioSep-SEC-s3000 (30 nm) and BioSep-SEC-s4000 (50 nm), as well as different extraction procedures were used to analyze the vital gluten samples. The ratio for EP to NEP was between 10.9 (G7) and 26.1 (G6). The ratio of SDSS proteins to GMP ranged from 4.6 (G21) to 21.6 (G26). Interestingly, the loading plot showed that SDSS proteins and NEP, as well as GMP and EP loaded in one direction, respectively, rather than vice versa as could be expected from the extraction procedures (Fig. 1). Since there was no significant correlation (p > 0.05) between both methods, it is recommended to use both to characterize the Mr distribution of vital gluten, because they provide complementary information, such as the Mr distribution.
Score plot (a) and loading plot (b) of analytical parameters of vital gluten samples G1-G39. Vital gluten samples from the same manufacturer are indicated by different symbols and colors. Ash content (AC), moisture content (MC), dry matter (DM), crude protein content (CP), lipid content (LC), starch content (SC), water absorption capacity (WAC), oil absorption capacity (OAC), relative and absolute (a) protein parameters gliadins (glia) and subtypes (ω5-gliadins, ω1,2-gliadins, α-gliadins, γ-gliadins), glutenins (glut) and their subunits high-molecular-weight (HMW)-glutenins, low-molecular-weight (LMW)-glutenins, glutenin macropolymer (GMP, HMW-GMP, LMW-GMP), SDS soluble proteins (SDSS), non-extractable proteins (NEP, HMW-NEP, LMW-NEP), extractable proteins (EP, HMW-EP, LMW-EP) and particle size distribution (D10, D50, D90)
NIRS
The NIR spectra of the vital gluten samples G1–G39 showed similarities in their curve profiles, only the intensities differed slightly (Fig. 2). Most variation occurred for the range of 5350–5200 cm−1, which is the typical band for the 2nd overtone of O–H bending [24] and most likely indicated differences in the moisture content of the vital gluten samples. Typical molecular vibrations for flour occurred in the region of 8220–7190 cm−1 for C–H 2nd overtone and combination modes, 7190–6720 cm−1 for O–H 1st overtone and C–H combination modes, 5700–5200 cm−1 for C=O stretching 2nd overtone and O–H combinations, 4400–4120 cm−1, 7150–6400 cm−1 for N–H stretching 1st overtone, 6400–5300 cm−1 for C–H stretching and combinations, as well as 5250–5040 cm−1 for O–H bending 2nd overtone [25]. NIRS was already used to successfully predict quality-related parameters in wheat [26] and was, therefore, considered to be suitable to predict quality parameters of vital gluten. The results of the area integration of the NIR spectra (each 50 cm−1) was assessed by PCA and showed that there were similarities between the vital gluten samples of the same manufacturer. However, many vital gluten samples from different suppliers were located in the same region, making it difficult to assign them to a specific manufacturer (Fig. 3). We assumed that the production processes varied from manufacturer to manufacturer and caused differences in fundamental characteristics that might explain the differences in functionality observed between the vital gluten samples [10]. Since we had no access to confidential information on specific process parameters, our study tried the reverse approach to collect as much structural and spectral data as possible and use this to assign vital gluten samples to different manufacturers. Based on the spectral analysis, samples G1, G31 and G36 stood out from the rest, but further studies would be necessary to identify the exact molecular structures responsible for the differences. All in all, NIRS seems to be a promising tool to characterize vital gluten, but further studies based on a much larger sample set will be necessary to establish a clear relationship between spectral characteristics and vital gluten composition.
CD spectroscopy
CD spectroscopy was used to determine secondary structural elements of vital gluten samples G1–G39. Figure 4 shows the far-UV spectra (below 250 nm) of gliadins, glutenins and gluten. At this wavelength range mainly the peptide bond absorbs, allowing conclusions about the secondary structure of proteins. The spectra had two characteristics of an α-helical conformation, expressed by two minima at 206–208 nm and 220–224 nm. Furthermore, the intensity of the spectra within one protein fraction was similar. However, larger variations occurred for glutenins, as well as smaller variations for gliadins and gluten at about 222 nm. This variance might be explained by the presence of disulfide bonds, as they have their maximum at 240–250 nm and can distort at about 224 nm [27]. CD spectroscopy is one way of looking at the secondary structural elements, but in the case of vital gluten, the spectra were too similar to identify differences between the samples. For this reason, it does not appear to provide further information that may be useful to characterize vital gluten.
Principal component analysis of all analytical parameters
PCA was performed with all analytical parameters (Fig. 1) and eight principle components (PC1–PC8) were considered to cover 86.4% of the whole data variance. The component matrix indicated that the individual principal components were influenced by various parameters resulting in comparatively low percentages of explained variance for PC1 (25.3%) and PC2 (21.9%). The loading plot (Fig. 1b) shows quite clearly that the content of α-, γ-, ω1,2- and ω5-gliadins had a positive impact on PC1, but a negative impact on PC2. Vice versa, LMW-GS and HMW-GS had a negative influence on PC1, but positive on PC2. EP- und GMP-related parameters as well as particle size distribution and water and crude protein content pointed to the top right corner, whereas SDSS protein- and NEP-related parameters together with ash and lipid content pointed to the bottom left corner. The score plot shows the distribution and the corresponding clustering of the vital gluten samples (Fig. 1a). Especially, G19–G22 were quite clearly separated from the rest of the samples, but they also showed greater variability within than the rest of the samples. G29–G33, as well as G12–G18 were located together in a comparatively narrow area and could be clearly assigned to a cluster. There were manufacturer-dependent similarities for the other vital gluten samples, but these could not be unambiguously allocated to a special manufacturer, because of overlapping clusters. To study whether the outstanding samples G19–G22 confounded the loadings and scores of the samples G1–G18 and G23–G39, another PCA was performed without these samples (Online Resource 1, Figure S1). PC1–PC8 covered 85.7% of the whole data variance, resulting in a coverage of 27.4% for PC1 and 16.8% for PC2. Overall, the loading plot put the same parameters together as before (Fig. 1b), but the influence on PC1 and PC2 changed, with a switch of gliadin- and glutenin-related parameters. In total, the samples G29–G33 clearly clustered together as before and the overall picture regarding the distribution of samples from the same manufacturer was comparable. In addition, various combinations were evaluated, such as removing either the relative or the absolute values for the content of the protein fractions, but all PCA plots revealed similar distributions of the vital gluten samples. A clustering was possible for some manufacturers, especially the one specializing in vital gluten production (G19–G22). For others, the PCA revealed clear similarities on the one hand, but on the other hand, the differences were not large enough to allow a clear assignment to one specific manufacturer. One additional limitation was that the origin of some samples were unknown since they were supplied by distributors, so that some samples might in fact be from one of the manufacturers already included. Due to the lack of information on specific proprietary production parameters, we could not establish clear relationships between the raw materials used, the process of gluten-starch separation and the fundamental characteristics of the commercial vital gluten samples reported here. Our observation that G19–G22 clearly differed from the other samples suggests that a manufacturing process tailored to producing vital gluten of high quality causes structural differences compared to those samples that are gained as a by-product of starch. Wheat of the highest baking quality typically goes directly into the production of bread, but would certainly also yield vital gluten of improved functionality, if used for this purpose. Further work will focus more specifically on relating process-specific parameters to the structural and functional properties of vital gluten.
Conclusion
Due to the increasing use of vital gluten for different food, feed and non-food applications, it is necessary to achieve a profound characterization of vital gluten samples and determine whether differences in composition are manufacturer dependent. The combination of all chemical analyses revealed that the samples from the manufacturer specializing in vital gluten production had a different composition and a greater variability compared to all other samples that were obtained as a co-product during wheat starch production. While our analyses revealed that samples from the same manufacturer were indeed similar in their composition, we also found that the range of variability over all samples was comparatively low. This makes it difficult to identify clear differences between the samples that can be related to the manufacturing process and also help explain differences in functionality.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
CODEX STAN 163-1987 (2001) Codex Standard for wheat protein products including wheat gluten
Bailey CH (1941) A translation of Beccari’s lecture ‘concerning grain’ (1728). Cereal Chem 18:555–561
Van Der Borght A, Goesaert H, Veraverbeke WS, Delcour JA (2005) Fractionation of wheat and wheat flour into starch and gluten: overview of the main processes and the factors involved. J Cereal Sci 41:221–237
Weegels PL, de Groot AMG, Verhoek JA, Hamer RJ (1994) Effects on gluten of heating at different moisture contents. II. Changes in physico-chemical properties and secondary structure. J Cereal Sci 19:39–47
Ortolan F, Steel CJ (2017) Protein characteristics that affect the quality of vital wheat gluten to be used in baking: a review. Compr Rev Food Sci Food Saf 16:369–381
Scherf KA, Wieser H, Koehler P (2016) Improved quantitation of gluten in wheat starch for celiac disease patients by gel-permeation high-performance liquid chromatography with fluorescence detection (GP-HPLC-FLD). J Agric Food Chem 64:7622–7631
Scherf KA, Koehler P, Wieser H (2016) Gluten and wheat sensitivities. J Cereal Sci 67:2–11
Delcour JA, Joye IJ, Pareyt B, Wilderjans E, Brijs K, Lagrain B (2012) Wheat gluten functionality as a quality determinant in cereal-based food products. Annu Rev Food Sci Technol 3:469–492
Day L, Augustin MA, Batey IL, Wrigley CW (2006) Wheat-gluten uses and industry needs. Trends Food Sci Technol 17:82–90
Schopf M, Scherf KA (2020) Predicting vital wheat gluten quality using the gluten aggregation test and the microscale extension test. Curr Res Food Sci 3:322–328
Kaushik R, Kumar N, Kumar Sihag M, Ray A (2015) Isolation, characterization of wheat gluten and its regeneration properties. J Food Sci Technol 52:5930–5937
Jakobi S, Jekle M, Becker T (2018) Direct link between specific structural levels of starch and hydration properties. Carbohyd Polym 181:159–166
Schopf M, Scherf KA (2018) Wheat cultivar and species influence variability of gluten ELISA analyses based on polyclonal and monoclonal antibodies R5 and G12. J Cereal Sci 83:32–41
van Eckert R, Berghofer E, Ciclitira PJ, Chirdo F, Denery-Papini S, Ellis HJ, Ferranti P, Goodwin P, Immer U, Mamone G, Méndez E, Mothes T, Novalin S, Osman A, Rumbo M, Stern M, Thorell L, Whim A, Wieser H (2006) Towards a new gliadin reference material-isolation and characterisation. J Cereal Sci 43:331–341
Batey IL, Gupta RB, MacRitchie F (1991) Use of size-exclusion high-performance liquid chromatography in the study of wheat flour proteins: an improved chromatographic procedure. Cereal Chem 68:207–209
Gupta RB, Khan K, MacRitchie F (1993) Biochemical basis of flour properties in bread wheats. I. Effects of variation in the quantity and size distribution of polymeric protein. J Cereal Sci 18:23–41
Sayaslan A, Seib PA, Chung OK (2010) Properties of starch and vital gluten isolated from wheat flour by three different wet-milling methods. J Appl Biol Sci 4:51–56
Ortolan F, Corrêa GP, Lopes da Cunha R, Steel CJ (2017) Rheological properties of vital wheat glutens with water or sodium chloride. LWT-Food Sci Technol 79:647–654
Belton PS (1999) Mini review—on the elasticity of wheat gluten. J Cereal Sci 29:103–107
Wadhawan CK, Bushuk W (1989) Studies on vitality of commercial gluten. I. Physical, chemical and technological characteristics. Cereal Chem 66:456–461
Wieser H, Antes S, Seilmeier W (1998) Quantitative determination of gluten protein types in wheat flour by reversed-phase high-performance liquid chromatography. Cereal Chem 75:644–650
Goesaert H, Brijs K, Veraverbeke WS, Courtin CM, Gebruers K, Delcour JA (2005) Wheat flour constituents: how they impact bread quality, and how to impact their functionality. Trends Food Sci Technol 16:12–30
Thanhaeuser SM, Wieser H, Koehler P (2014) Correlation of quality parameters with the baking performance of wheat flours. Cereal Chem 91:333–341
Li Vigni M, Durante C, Foca G, Marchetti A, Ulrici A, Cocchi M (2009) Near infrared spectroscopy and multivariate analysis methods for monitoring flour performance in an industrial bread-making process. Anal Chim Acta 642:69–76
Pojić MM, Mastilović JS (2013) Near infrared spectroscopy—advanced analytical tool in wheat breeding, trade, and processing. Food Bioprocess Technol 6:330–352
Dowell FE, Maghirang EB, Xie F, Lookhart GL, Pierce RO, Seabourn BW, Bean SR, Wilson JD, Chung OK (2006) Predicting wheat quality characteristics and functionality using near-infrared spectroscopy. Cereal Chem 83:529–536
Tatham AS, Field JM, Smith SJ, Shewry PR (1987) The conformations of wheat gluten proteins. II. Aggregated gliadins and low molecular weight subunits of glutenin. J Cereal Sci 5:203–214
Acknowledgements
The authors gratefully acknowledge Prof. Dr. Johannes Buchner and Dr. Pamina Kazman (TUM Chair of Biotechnology) for providing access to the Chirascan Plus instrument.
Funding
Open Access funding enabled and organized by Projekt DEAL. This IGF Project of the FEI was supported via AiF within the programme for promoting the Industrial Collective Research (IGF) of the German Ministry of Economic Affairs and Energy (BMWi), based on a resolution of the German Parliament. Project 19710 N.
Author information
Authors and Affiliations
Contributions
Conceptualization: T.B., M.J., K.A.S.; methodology, validation, formal analysis, investigation: M.S., M.C.W.; resources: T.B., M.J., K.A.S.; writing—original draft, visualization: M.S.; writing—review and editing: M.C.W., T.B., M.J., K.A.S.; supervision, project administration, funding acquisition: T.B., M.J., K.A.S.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schopf, M., Wehrli, M.C., Becker, T. et al. Fundamental characterization of wheat gluten. Eur Food Res Technol 247, 985–997 (2021). https://doi.org/10.1007/s00217-020-03680-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03680-z