Abstract
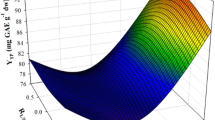
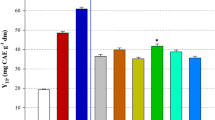
In this study, the extraction of polyphenols from Moringa oleifera leaves was investigated, using a biomolecule-based low-transition temperature mixture (LTTM), composed of glycerol and sodium acetate. The first step was to optimise LTTM concentration (C LTTM) and the molar ratio (R mol) of glycerol-to-sodium acetate, by employing a Box–Behnken experimental design. Following this, a kinetic assay was undertaken to assess the effect of temperature. A maximum yield in total polyphenols (Y TP = 53.80 mg gallic acid equivalents per g lyophilised material) was achieved using a C LTTM = 80% (w/v) and a R mol = 6. The extraction followed a second-order model, requiring activation energy (E a) of 55.71 kJ mol−1. Comparative evaluation using 80% ethanol showed that the LTTM used was significantly more efficient in extracting polyphenols and flavonoids, yielding extracts with higher reducing power. However, results concerning the antiradical activity were contradictory. Liquid chromatography–diode array–mass spectrometry examination of both the LTTM and the hydroalcoholic extracts revealed some qualitative differences, which might indicate selectivity of the LTTM towards relatively polar substances.






Similar content being viewed by others
Abbreviations
- A AR :
-
Antiradical activity (μmol DPPH g−1)
- C LTTM :
-
LTTM concentration (%, w/v)
- E a :
-
Activation energy (kJ mol−1)
- h :
-
Initial extraction rate (mg g−1 min−1)
- k :
-
Second-order extraction rate constant (g mg−1 min−1)
- P R :
-
Reducing power (μmol AAE g−1)
- R L/S :
-
Liquid-to-solid ratio (mL g−1)
- R mol :
-
HBD/HBA molar ratio (dimensionless)
- t :
-
Time (min)
- T :
-
Temperature (°C or K)
- Y TFn :
-
Yield in total flavonoids (mg RtE g−1)
- Y TP :
-
Yield in total polyphenols (mg GAE g−1)
- Y TP(s) :
-
Yield in total polyphenols at saturation (mg GAE g−1)
- AAE:
-
Ascorbic acid equivalents
- DES:
-
Deep eutectic solvents
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl radical
- GAE:
-
Gallic acid equivalents
- HBA:
-
Hydrogen bond acceptor
- HBD:
-
Hydrogen bond donor
- LTTM:
-
Low-transition temperature mixture
- MoL:
-
M. oleifera leaves
- MW:
-
Molecular weight
- RtE:
-
Rutin equivalents
- TPTZ:
-
2,4,6-Tripyridyl-s-triazine
References
Anwar F, Latif S, Ashraf M, Gilani AH (2007) Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res 21:17–25
Ferreira PMP, Farias DF, Oliveira JTdA, Carvalho AdFU (2008) Moringa oleifera: bioactive compounds and nutritional potential. Rev Nutr 21:431–437
Farooq F, Rai M, Tiwari A, Khan AA, Farooq S (2012) Medicinal properties of Moringa oleifera: an overview of promising healer. J Med Plants Res 6:4368–4374
Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S (2015) Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Int J Mol Sci 16:12791–12835
Shih M-C, Chang C-M, Kang S-M, Tsai M-L (2011) Effect of different parts (leaf, stem and stalk) and seasons (summer and winter) on the chemical compositions and antioxidant activity of Moringa oleifera. Int J Mol Sci 12:6077–6088
Sreelatha S, Padma P (2009) Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Human Nutr 64:303–311
Pakade V, Cukrowska E, Chimuka L (2013) Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. South African J Sci 109:1–5
Verma AR, Vijayakumar M, Mathela CS, Rao CV (2009) In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem Toxicol 47:2196–2201
Luqman S (2012) Ferric reducing antioxidant power and free radical scavenging activity of Moringa oleifera: Relevance in oxidative stress. Evidence-Based Compl Alter Med Article ID 519084
Ndhlala AR, Mulaudzi R, Ncube B, Abdelgadir HA, du Plooy CP, Van Staden J (2014) Antioxidant, antimicrobial and phytochemical variations in thirteen Moringa oleifera Lam. cultivars. Molecules 19:10480–10494
Iqbal S, Bhanger M (2006) Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J Food Compos Anal 19:544–551
Vongsak B, Sithisarn P, Mangmool S, Thongpraditchote S, Wongkrajang Y, Gritsanapan W (2013) Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind Crops Prod 44:566–571
Matshediso PG, Cukrowska E, Chimuka L (2015) Development of pressurised hot water extraction (PHWE) for essential compounds from Moringa oleifera leaf extracts. Food Chem 172:423–427
Rodríguez-Pérez C, Gilbert-López B, Mendiola JA, Quirantes-Piné R, Segura-Carretero A, Ibáñez E (2016) Optimization of microwave-assisted extraction and pressurized liquid extraction of phenolic compounds from Moringa oleifera leaves by multiresponse surface methodology. Electrophoresis 37:1938–1946
Barba FJ, Terefe NS, Buckow R, Knorr D, Orlien V (2015) New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Res Inter 77:725–742
Barba FJ, Parniakov O, Pereira SA, Wiktor A, Grimi N, Boussetta N, Saraiva JA, Raso J, Martin-Belloso O, Witrowa-Rajchert D, Lebovka N, Vorobiev E (2015) Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res Inter 77:773–798
Paiva A, Craveiro R, Aroso I, Martins M, Reis RL, Duarte ARC (2014) Natural deep eutectic solvents–solvents for the 21st century. Sustain Chem Eng 2:1063–1071
Dai Y, Witkamp G-J, Verpoorte R, Choi YH (2013) Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal Chem 85:6272–6278
Mouratoglou E, Malliou V, Makris DP (2016) Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valor 7:1377–1387
Makris DP (2015) A novel kinetic assay for the examination of solid-liquid extraction of flavonoids from plant material. Res J Chem Sci 5:18–23
Makris DP (2016) Kinetics of ultrasound-assisted flavonoid extraction from agri-food solid wastes using water/glycerol mixtures. Resources 5:7
Kanakaki E, Siderakou D, Kallithraka S, Kotseridis Y, Makris DP (2015) Effect of the degree of toasting on the extraction pattern and profile of antioxidant polyphenols leached from oak chips in model wine systems. Eur Food Res Technol 240:1065–1074
Shehata E, Grigorakis S, Loupassaki S, Makris DP (2015) Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Separ Purif Technol 149:462–469
Cui Q, Peng X, Yao X-H, Wei Z-F, Luo M, Wang W, Zhao C-J, Fu Y-J, Zu Y-G (2015) Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Separ Purif Technol 150:63–72
Wei Z-F, Wang X-Q, Peng X, Wang W, Zhao C-J, Zu Y-G, Fu Y-J (2015) Fast and green extraction and separation of main bioactive flavonoids from Radix scutellariae. Ind Crops Prod 63:175–181
Yao X-H, Zhang D-Y, Duan M-H, Cui Q, Xu W-J, Luo M, Li C-Y, Zu Y-G, Fu Y-J (2015) Preparation and determination of phenolic compounds from Pyrola incarnata Fisch. with a green polyols based-deep eutectic solvent. Separ Purif Technol 149:116–123
Zhang Q, Vigier KDO, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108–7146
Bi W, Tian M, Row KH (2013) Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J Chrom A 1285:22–30
Chan C-H, Yusoff R, Ngoh G-C (2014) Modeling and kinetics study of conventional and assisted batch solvent extraction. Chem Eng Res Des 92:1169–1186
Bucić-Kojić A, Sovová H, Planinić M, Tomas S (2013) Temperature-dependent kinetics of grape seed phenolic compounds extraction: experiment and model. Food Chem 136:1136–1140
Krishnan RY, Rajan K (2016) Microwave assisted extraction of flavonoids from Terminalia bellerica: Study of kinetics and thermodynamics. Separ Purif Technol 157:169–178
Philippi K, Tsamandouras N, Grigorakis S, Makris DP (2016) Ultrasound-assisted green extraction of eggplant peel (Solanum melongena) polyphenols using aqueous mixtures of glycerol and ethanol: optimisation and kinetics. Environ Proc 3:369–386
Tzima K, Kallithraka S, Kotseridis Y, Makris DP (2014) Kinetic modelling for flavonoid recovery from red grape (Vitis vinifera) pomace with aqueous lactic acid. Processes 2:901–911
Makris DP, Kefalas P (2015) Kinetic modelling for polyphenol extraction from onion (Allium cepa) solid wastes using acidified water/ethanol mixture. Acta Alimen 44:482–492
Cacace J, Mazza G (2003) Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J Food Sci 68:240–248
Garofulić IE, Dragović-Uzelac V, Jambrak AR, Jukić M (2013) The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. Marasca). J Food Eng 117:437–442
Cuevas-Valenzuela J, González-Rojas Á, Wisniak J, Apelblat A, Pérez-Correa JR (2014) Solubility of (+)-catechin in water and water-ethanol mixtures within the temperature range 277.6–331.2 K: fundamental data to design polyphenol extraction processes. Fluid Phase Equil 382:279–285
Karakashov B, Grigorakis S, Loupassaki S, Makris DP (2015) Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology. J Applied Res Med Arom Plants 2:1–8
Nascimento JA, Araújo KL, Epaminondas PS, Souza AS, Magnani M, Souza AL, Soledade LE, Queiroz N, Souza AG (2013) Ethanolic extracts of Moringa oleifera Lam. J Thermal Anal Calor 114:833–838
Michail A, Sigala P, Grigorakis S, Makris DP (2015) Kinetics of ultrasound-assisted polyphenol extraction from spent filter coffee using aqueous glycerol. Chem Eng Comm 203:407–413
Sadek ES, Makris DP, Kefalas P (2009) Polyphenolic composition and antioxidant characteristics of kumquat (Fortunella margarita) peel fractions. Plant Foods Human Nutr 64:297–302
Apostolakis A, Grigorakis S, Makris DP (2014) Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Separ Purif Technol 128:89–95
Tang B, Row KH (2013) Recent developments in deep eutectic solvents in chemical sciences. Monatsh Chem-Chem Monthly 144:1427–1454
Pandey A, Rai R, Pal M, Pandey S (2014) How polar are choline chloride-based deep eutectic solvents? Phys Chem Chem Phys 16:1559–1568
Bakirtzi C, Triantafyllidou K, Makris DP (2016) Novel lactic acid-based natural deep eutectic solvents: efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J Applied Res Med Arom Plants 3:120–127
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Karageorgou, I., Grigorakis, S., Lalas, S. et al. Enhanced extraction of antioxidant polyphenols from Moringa oleifera Lam. leaves using a biomolecule-based low-transition temperature mixture. Eur Food Res Technol 243, 1839–1848 (2017). https://doi.org/10.1007/s00217-017-2887-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2887-1