Abstract
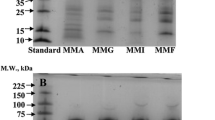
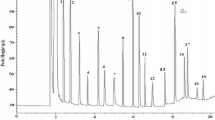
This study investigates the properties of globular tiger nut proteins by determining the average molecular mass, protein fractions, denaturation temperature and the isoelectric point. Tiger nut proteins (TNP) prepared by aqueous extraction had a purity of 83.5 % w/w on dry mass. Fractionation of TNP revealed that glutelin was the constituent with the highest concentration (>47.5 % w/w), followed by albumin (31.8 %), globulin (4.7 %) and prolamin (3.8 %). A residual protein fraction of 12.2 % appeared as electrophoretically similar to glutelin. TNP had an apparent molecular mass (m m) of 5.3–88 kDa. Both albumin and globulin fractions had m m of 5.3–73 kDa, whilst prolamin and glutamine fractions were 5.3–46.2 and 5.3–30 kDa, respectively. TNP had an onset temperature (T o), denaturation temperature (T d) and enthalpy of transition (∆H) of 54.40, 69.61 °C and 5.43 J/g, respectively. In decreasing order of T o and T d among the protein fractions were prolamin, albumin, globulin and glutelin. Albumin and glutelin showed the highest and lowest ∆H, respectively. Finally, the isoelectric point of TNP was 4.90. Fundamentally, the properties of TNP show that they might be useful for the techno-functional application in tiger nut-based food systems.



Similar content being viewed by others
References
Cortés C, Esteve MJ, Frígola A, Torregrosa F (2004) Physical and chemical properties of different commercially available types of “horchata de chufa”. Ital J Food Sci 16:113–121
Coşkuner Y, Ercan R, Karababa E, Nazlıcan AN (2002) Physical and chemical properties of chufa (Cyperus esculentus L.) tubers grown in the Çukurova region of Turkey. J Sci Food Agric 82:625–631
Belewu MA (2008) Preparation of Kunnu from unexploited rich food source: tiger nut (Cyperus esculentus). Pak J Nutr 7:109–111
Akoma O, Elekwa UO, Afodunrinbi AT, Onyeukwu GC (2000) Yogurt from coconut and tiger nuts. J Food Technol Afr 5:132–134
Sanful RE (2009) Production and sensory evaluation of tiger nut beverages. Pak J Nutr 8:688–690
Kizzie-Hayford N, Jaros D, Schneider Y, Rohm H (2015) Characteristics of tiger nut milk: effects of milling. Int J Food Sci Technol 50:381–388
Bosch L (2005) RP-HPLC determination of tiger nut and orgeat amino acid contents. Food Sci Technol Int 11:33–40
Sánchez-Zapata E, Fernández-López J, Angel Pérez-Alvarez J (2012) Tiger nut (Cyperus esculentus) commercialization: health aspects, composition, properties, and food applications. Compr Rev Food Sci Food Saf 11:366–377
Siebert KJ (2003) Modeling protein functional properties from amino acid composition. J Agric Food Chem 51:7792–7797
Deng Q, Wang L, Wei F, Xie B, Huang F, Huang W, Shi J, Huang Q, Tian B, Xue S (2011) Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem 124:1458–1465
Shabbir MA, Anjum FM, Zahoor T, Nawaz H (2011) Electrophoretic and functional mapping of indica rice glutelin protein isolates. Int J Food Prop 14:1375–1385
Sikorski ZE (2001) Chemical and functional properties of food proteins. CRC Press, Boca Raton
Wingfield P (2001) Current protocols in protein science. Wiley, Maryland
Matsudaira PT (1993) A practical guide to protein and peptide purification for microsequencing. Academic Press, San Diego
Wessel D, Flügge UI (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 138:141–143
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Rabilloud T, Carpentier G, Tarroux P (1988) Improvement and simplification of low-background silver staining of proteins by using sodium dithionite. Electrophoresis 9:288–291
Chiu MH, Prenner EJ (2011) Differential scanning calorimetry: an invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J Pharm Bioallied Sci 3:39–59
Salgın S, Salgın U, Bahadır S (2012) Zeta Potentials and isoelectric points of biomolecules: the effects of ion types and ionic strengths. Int J Electrochem Sci 7:12404–12414
Manamperi WAR, Wiesenborn DP, Chang SKC, Pryor SW (2011) Effects of protein separation conditions on the functional and thermal properties of canola protein isolates. J Food Sci 76:266–273
Maloney KP, Truong V-D, Allen JC (2012) Chemical optimization of protein extraction from sweet potato (Ipomoea batatas) peel. J Food Sci 77:E307–E312
Skoch LV, Deyoe CW, Shoup FK, Bathurst J, Liang D (1970) Protein fractionation of sorghum grain. Cereal Chem pp 472–482
Lisińska G (1989) Potato science and technology. Elsevier Science Pub. Co., London
El-Shenawy M, Abd El-Aziz M, El-kholy WI, Fouad MT (2012) Probiotic yoghurt manufactured with tiger-nut extract (Cyperus esculentus) as a functional dairy food. J Agric Res Nat Resour 1:20–31
Nehete J, Narkhede M, Bhambar R, Gawali S (2013) Natural proteins: sources, isolation, characterization and applications. Pharmacogn Rev 7:107
Leubner-Metzger G, Meins F (1999) In: Datta SK, Muthukrishnan S (eds) Pathogenesis-related proteins in plants. CRC Press, Florida
Bhambhani A, Kumar CV (2007) In: Basbanes LV (ed) Advanced materials research trends. Nova Science Publishers, Inc., New York
Urry DW, Luan CH, Parker TM, Gawda DC, Prasad KU, Reid MC, Safavy A (1991) Temperature of polypeptide inverse temperature transition depends on mean residue hydrophobicity. J Am Chem Soc 113:4346–4348
Zayas JF (1997) Emulsifying properties of proteins. Springer, Berlin
Pots AM, de Jongh HHJ, Gruppen H, Hamer RJ, Voragen AGJ (1998) Heat-induced conformational changes of patatin, the major potato tuber protein. Eur J Biochem 252:66–72
Makhatadze GI (2001) Measuring protein thermostability by differential scanning calorimetry. Curr Protoc Protein Sci 7.9.1–14
Ju ZY, Hettiarachchy NS, Rath N (2001) Extraction, denaturation and hydrophobic properties of rice flour proteins. J Food Sci 66:229–232
Gill P, Moghadam TT, Ranjbar B (2010) Differential scanning calorimetry techniques: applications in biology and nanoscience. J Biomol Tech 21:167–193
Demarest DJ, Verna F (2015) In: Houde DJ, Berkonitz SA (eds) Biophysical characterisation of proteins in developing biopharmaceuticals, 1st edn. Elsevier, Amsterdam
Warner RC (1942) The alkaline hydrolysis of egg albumin. J Biol Chem 142:741–756
Pearsall WH, Ewing J (1924) The isoelectric points of some plant proteins. Biochem J 18:329–339
Henriksson G, Englund AK, Johansson G, Lundahl P (1995) Calculation of the isoelectric points of native proteins with spreading of pKa values. Electrophoresis 16:1377–1380
Bryan WP (1978) The isoionic point of amino acids and proteins. Biochem Educ 6:14–15
Vaclavik V, Christian EW (2014) Essentials of food science. Springer, Texas
Acknowledgments
Nazir Kizzie-Hayford receives support through a joint scholarship by the Government of Ghana, Ministry of Education (GOG-MoE) and the German Academic Exchange Services (DAAD), Grant number A/12/97408. We are grateful to Mr André Kupka, Institute for Process Engineering and Environmental Technology, Technische Universität Dresden, Germany, for technical assistance with zeta potential measurement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with Ethics Requirements
This study does not contain any experiment involving human or animal subjects.
Rights and permissions
About this article
Cite this article
Kizzie-Hayford, N., Jaros, D., Schneider, Y. et al. Physico-chemical properties of globular tiger nut proteins. Eur Food Res Technol 241, 835–841 (2015). https://doi.org/10.1007/s00217-015-2508-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2508-9