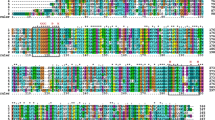
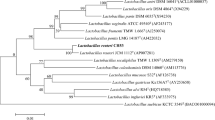
Abstract
The genes involved in the glycerol metabolism, glycerol dehydratase (gdh) and two propanediol dehydrogenases (pdh30 and pdh1734), were analyzed in different reuterin- and non-reuterin-producing lactobacilli of biotechnological interest. All the reuterin-producing lactobacilli expressed the gdh, pdh30 and pdh1734, except Lb. coryniformis CECT 5711 which did not contain pdh30. Reuterin production levels in Lb. coryniformis CECT 5711 were much lower than those in reuterin-producing Lb. reuteri. A positive relationship between cobalamin production levels and reuterin production levels was observed in all reuterin-producing lactobacilli tested. Intriguingly, when Lb. coryniformis CECT 5711 was supplemented with cobalamin, a seven times increase in reuterin production was observed. On the other hand, Lb. brevis ESI38 that possess and express gdh, pdh30 and pdh1734, was unable to produce reuterin or cobalamin. To study the role of pdh30 during glycerol metabolism, the gene disruption mutant Lb. brevis INIA ESI38::pORI28-pdh30 was constructed. HPLC analysis of the glycerol fermentation products showed an involvement of the pdh30 in the 3-hydroxypropionic acid (3-HP) biosynthesis. However, Lb. coryniformis, that lack pdh30, showed the higher levels of 3-HP, indicating other catalytic mechanisms to produce 3-HP in this strain. The 1,3-propanediol peak was detected in the Lb. reuteri and Lb. coryniformis chromatograms, but not in Lb. brevis, which also confirm divergences in Lactobacillus glycerol metabolism.





Similar content being viewed by others
References
Reuter G (1965) Das vorkommen von Laktobazillen in lebensmitteln und ihr verhalten in menschlichen intestinal trakt. Zbl Bak Parasit Infec Hyg Orig 197:468–487
Vogel RF, Bocker G, Stolz P, Ehrmann M, Fanta D, Ludwig W, Pot B, Kersters K, Schleifer KH, Hammes WP (1994) Identification of lactobacilli from sourdough and description of Lactobacillus pontis sp. nov. Int J Syst Bacteriol 44:223–229
Casas I, Dobrogosz WJ (2000) Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microb Ecol Health Dis 12:247–285
Francavilla R, Lionetti E, Castellaneta S, Ciruzzi F, Indrio F, Masciale A, Fontana C, La Rosa MM, Cavallo L, Francavilla A (2012) Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhea—a double-blind study. Aliment Pharmacol Ther 36:363–369
Axelsson T, Chung TC, Dobrogosz WJ, Lindgren SE (1989) Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb Ecol Health Dis 2:131–136
Talarico TL, Dobrogosz WJ (1990) Purification and characterization of glycerol dehydratase from Lactobacillus reuteri. Appl Environ Microbiol 56:1195–1197
Vollenweider S, Grassi G, König I, Puhan Z (2003) Purification and structural characterization of 3-hydroxypropionaldehyde and its derivates. J Agric Food Chem 51:3287–3293
Stevens M, Vollenweider S, Lacroix C (2011) The potential of reuterin produced by Lactobacillus reuteri as a broad spectrum preservative in food. In: Lacroix C (ed) Protective cultures, antimicrobial metabolites and bacteriophages for food and beverage biopreservation. Woodhead Publishing Limited, Cambridge, pp 129–160
El-Ziney MG, Debevere JM (1998) The effect of reuterin on Listeria monocytogenes and Escherichia coli O157:H7 in milk and cottage cheese. J Food Prot 61:1275–1280
El-Ziney MG, van den Tempel T, Debevere JM, Jakobsen M (1999) Application of reuterin produced by Lactobacillus reuteri 12002 for meat decontamination and preservation. J Food Prot 62:257–261
Arqués JL, Fernández J, Gaya P, Nuñez M, Rodríguez E, Medina M (2004) Antimicrobial activity of reuterin in combination with nisin against food-borne pathogens. Int J Food Microbiol 95:225–229
Arqués JL, Rodríguez E, Nuñez M, Medina M (2008) Inactivation of Gram-negative pathogens in refrigerated milk by reuterin in combination with nisin or the lactoperoxidase system. Eur Food Res Technol 227:77–82
Arqués JL, Rodríguez E, Nuñez M, Medina M (2008) Antimicrobial activity of nisin, reuterin, and the lactoperoxidase system on Listeria monocytogenes and Staphylococcus aureus in cuajada, a semisolid dairy product manufactured in Spain. J Dairy Sci 91:70–75
Gómez N, Ávila M, Gaya P, Garde S (2014) Prevention of late blowing defect by reuterin produced in cheese by a Lactobacillus reuteri adjunct. Food Microbiol 42:82–88
Montiel R, Martín-Cabrejas I, Langa S, El Aouad N, Arqués JL, Reyes F, Medina M (2014) Antimicrobial activity of reuterin produced by Lactobacillus reuteri on Listeria monocytogenes in cold-smoked salmon. Food Microbiol 44:1–5
Morita H, Toh H, Fukuda S, Horikawa H, Oshima K, Suzuki T, Murakami M, Hisamatsu S, Kato Y, Takizawa T, Fukuoka H, Yoshimura T, Itoh K, O’Sullivan DJ, McKay LL, Ohno H, Kikuchi J, Masaoka T, Hattori M (2008) Comparative genome analysis of Lactobacillus reuteri and Lactobacillus fermentum reveal a genomic island for reuterin and cobalamin production. DNA Res 15:151–161
Sriramulu DD, Liang M, Hernandez-Romero D, Raux-Deery E, Lunsdorf H, Parsons JB, Warren MJ, Prentice MB (2008) Lactobacillus reuteri DSM 20016 produces cobalamin dependent diol dehydratase in metabolosomes and metabolizes 1,2-propanediol by disproportionation. J Bacteriol 190:4559–4567
Abeles RH, Lee HA (1961) An intramolecular oxidation-reduction requiring a cobamide coenzyme. J Biol Chem 236:2347–2350
Smiley KL, Sobolov MA (1962) Cobamide-requiring glycerol dehydrase from an acrolein-forming Lactobacillus. Arch Biochem Biophys 97:538–543
Daniel R, Bobik TA, Gottschalk G (1998) Biochemistry of coenzyme B12-dependent glycerol and diol dehydratases and organization of the encoded genes. FEMS Microbiol Rev 25:553–566
Voisenet ME (1914) Sur un ferment, contenu dans les eaux, agente déshydratation de la glycérine. C R Acad Sci 150:1614–1616
Slininger PJ, Bothast RJ (1983) Optimizing aerobic conversion of glycerol to 3-hydroxypropionaldehyde. Appl Environ Microbiol 50:1444–1450
Seyfried MR, Daniel R, Gottschalk G (1996) Cloning, sequencing and overexpression of the genes encoding coenzyme B12-dependent glycerol dehydratase of Citrobacter freundii. J Bacteriol 178:5793–5796
Barbirato F, Soucaille P, Bories A (1996) Physiologic mechanisms involved in accumulation of 3-hydroxypropionaldehyde during fermentation of glycerol by Enterobacter agglomerans. Appl Environ Microbiol 42:4405–4409
Macis L, Daniel R, Gottschalk G (1998) Properties and sequence of the coenzyme B12-dependent glycerol dehydratase of Clostridium pasterianum. FEMS Microbiol Lett 164:21–28
Bobik TA, XuY Jeter RM, Otto KE, Roth JR (1997) Propanediol utilization genes (pdu) of Salmonella typhimurium: three genes for the propanediol dehydratase. J Bacteriol 179:6633–6639
Thiman KV (1957) The life of bacteria their growth, metabolism, and relationships. MacMillan, New York, pp xviii 4 + 775
Biebl H, Menzel K, Zeng AP, Deckwer WD (1987) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52:289–297
Zeng AP, Biebl H (2002) Bulk chemicals from biotechnology: the case of 1,3 propanediol production and the new trends. Adv Biochem Eng Biotechnol 74:239–259
Schütz H, Radler F (1984) Anaerobic reduction of glycerol to propanediol-1.3 by Lactobacillus brevis and Lactobacillus buchneri. Syst Appl Microbiol 5:169–178
Sauvageot N, Gouffi K, Laplace JM, Auffray Y (2000) Glycerol metabolism in Lactobacillus collinoides: production of 3-hydroxypropionaldheyde, a precursor of acrolein. Int J Food Microbiol 55:167–170
Rodríguez E, Arqués JL, Rodríguez R, Nuñez M, Medina M (2003) Reuterin production by lactobacilli isolated from pig faeces and evaluation of probiotics traits. Lett Appl Microbiol 37:259–263
Spinler JK, Taeweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J (2008) Human-derived Probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 14:166–171
Spinler JK, Sontakke A, Hollister EB, Venable SF, Oh PL, Balderas MA, Saulnier DM, Mistretta TA, Devaraj S, Walter J, Versalovic J, Highlander SK (2014) From prediction to function using evolutionary genomics: human-specific ecotypes of Lactobacillus reuteri have diverse probiotic functions. Genome Biol Evol 6:1772–1789
Martín R, Olivares M, Marín ML, Xaus J, Fernández L, Rodríguez JM (2005) Characterization of a reuterin-producing Lactobacillus coryniformis strain isolated from a goat’s milk cheese. Int J Food Microbiol 104:267–277
Marçal D, Rêgo AT, Carrondo MA, Enguita FJ (2009) 1,3-Propanediol dehydrogenase from Klebsiella pneumoniae: decameric quaternary structure and possible subunit cooperativity. J Bacteriol 191:1143–1151
Saulnier DM, Santos F, Roos S, Mistretta T-A, Spinler JK, Molenaar D, Teusink B, Versalovic J (2011) Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS ONE 6:18783
Stevens MJ, Vollenweider S, Meile L, Lacroix C (2011) 1,3-Propanediol dehydrogenases in Lactobacillus reuteri: impact on central metabolism and 3-hydroxypropionaldehyde production. Microb Cell Fact 10:61
Talarico TL, Casas IA, Chung TC, Dobrogosz WJ (1989) Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob Agents Chemother 32:1854–1858
Dishisha TL, Pereyra P, Pyo S-H, Britton RA, Hatti-Kaul R (2014) Flux analysis of the Lactobacillus reuteri propanediol-utilization pathway for production of 3-hydroxypropionaldehyde, 3-hydroxypropionic acid and 1, 3-propanediol from glycerol. Microb Cell Fact 13:76
Luo LH, Kim CH, Heo SY, Oh BR, Hong WK, Kim S, Kim DH, Seo JW (2012) Production of 3-hydroxypropionic acid through propionaldehyde dehydrogenase PduP mediated biosynthetic pathway in Klebsiella pneumonia. Bioresour Technol 103:1–6
Martens JH, Barg H, Warren MJ, Jahn D (2002) Microbial production of vitamin B12. Appl Microbiol Biotechnol 58:275–285
Taranto MP, Vera JL, Hugenholtz J, de Valdez GF, Sesma F (2003) Lactobacillus reuteri CRL1098 produces cobalamin. J Bacteriol 185:5643–5647
Vannini V, Rodríguez A, Vera JL, de Valdéz GF, Taranto MP, Sesma F (2011) Cloning and heterologous expression of Lactobacillus reuteri uroporphyrinogen III synthase/metyltransferase gene (cobA/hemD): preliminary characterization. Biotechnol Lett 33:1625–1632
Santos F, Vera JL, Van Der Heiden R, Valdéz G, De Vos WM, Sesma F, Hugenholtz J (2008) The complete coenzyme B12 biosynthesis gene cluster of Lactobacillus reuteri CRL 1098. Microbiology 154:81–93
Santos F, Vera JL, Lamosa P, de Valdez GF, de Vos WM, Santos H, Sesma F, Hugenholtz J (2007) Pseudovitamin B12 is the corrinoid produced by Lactobacillus reuteri CRL1098 under anaerobic conditions. FEBS Lett 581:4865–4870
Vera JL (2007) Biosíntesis de cobalamina en Lactobacillus reuteri CRL1098. Doctoral Thesis, Universidad Nacional de Tucumán (Argentina), Tucumán, Argentina
Rodriguéz E, Arqués JL, Rodríguez R, Peirotén A, Landete JM, Medina M (2012) Antimicrobial properties of probiotic strains isolated from breast-fed infants. J Funct Foods 4:542–551
Cogan TM, Barbosa M, Beuvier E, Bianchi-Salvadori B, Cocconcelli PS, Fernandes I, Gomez J, Kalantzopoulos G, Ledda A, Medina M, Rea MC, Rodríguez E (1997) Characterization of the lactic acid bacteria in artisanal dairy products. J Dairy Res 64:409–421
Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MWEJ, Stiekema W, Klein Lankhorst RM, Bron PA, Hoffer SM, Nierop Groot MN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ (2003) Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci USA 100:1990–1995
Circle SJ, Stone L, Boruff CS (1945) Acrolein determination by means of tryptophane. Ind Engine Chem 17:259–262
Horwitz W (2000) Official methods of analysis of AOAC International. AOAC International, Gaithersburg
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Claisse O, Lonvaud-Funel A (2001) Primers and a specific DNA probe for detecting lactic acid bacteria producing 3-hydroxypropionaldehyde from glycerol in spoiled ciders. J Food Prot 64:833–837
Ruiz-Barba JL, Maldonado A, Jiménez-Díaz R (2005) Small-scale total DNA extraction from bacteria and yeast for PCR applications. Anal Biochem 15:333–335
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Russell WM, Klaenhammer TR (2001) Efficient system for directed integration into the Lactobacillus acidophilus and Lactobacillus gasseri chromosomes via homologous recombination. Appl Environ Microbiol 67:4361–4364
Maguin E, Duwat P, Hege T, Ehrlich D, Gruss A (1992) New thermosensitive plasmid for gram-positive bacteria. J Bacteriol 174:5633–5638
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Langa S, Landete JM, Martín-Cabrejas I, Rodríguez E, Arqués JL, Medina M (2013) In situ reuterin production by Lactobacillus reuteri in dairy products. Food Control 33:200–206
Sauvageot N, Muller C, Hartke A, Auffray Y, Laplace JM (2002) Characterisation of the diol dehydratase pdu operon of Lactobacillus collinoides. FEMS Microbiol Lett 209:69–74
Lawrence JG (2003) Gene organization: selection, selfishness, and serendipity. Ann Rev Microbiol v57:419–440
Glaser P, Frangeul L, Buchrieser C, Rusniok C, Amend A, Baquero F, Berche P, Bloecker H, Brandt P et al (2001) Comparative genomics of Listeria species. Science 294:849–852
McClelland MK, Sanderson E, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M et al (2001) Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852–856
Thomson NR, Howard S, Wren BW, Holden MT, Crossman L, Challis GL, Churcher C, Mungall K, Brooks K et al (2006) The complete genome sequence and comparative genome analysis of the high pathogenicity Yersinia enterocolitica strain 8081. PLoS Genet 2:206
Toraya T, Shirakashi T, Kosuga T, Fukui S (1976) Substrate specificity of coenzyme B12-dependent diol dehydrase: glycerol as both a good substrate and a potent inactivator. Biochem Biophys Res Commun 22:475–480
Acknowledgments
We are grateful to Dr. R. Muñoz and Dr. J.M. Rodríguez for kindly providing us with some of the strains used in this study. This work was supported by project RTA 2010-00116-00-00 and RC2010-06925 from the Spanish Ministry of Economy and Competitiveness (MINECO). Dr. Landete has a postdoctoral contract of the program “Ramón y Cajal” (MINECO, Spain).
Conflict of interest
None.
Compliance with Ethics requirement
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Langa, S., Arqués, J.L., Gaya, P. et al. Glycerol and cobalamin metabolism in lactobacilli: relevance of the propanediol dehydrogenase pdh30. Eur Food Res Technol 241, 173–184 (2015). https://doi.org/10.1007/s00217-015-2443-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2443-9