Abstract
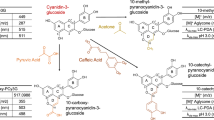
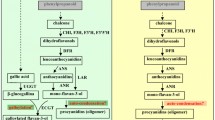
The degradation behaviors of four different proanthocyanidins dimers including B-type (e)picatechin (E)C dimer and A-type (epi)catechin (E)C dimer, A-type (epi)catechin-3-O-gallate (E)CG dimer and A-type (epi)gallocatechin-3-O-gallate (E)GCG dimer as affected by pH and temperature were compared in this study. The results showed that the stability of proanthocyanidins dimers was not only temperature and pH dependent, but also structure dependent. Proanthocyanidins dimers were found to be unstable at pH as low as 1.5 and physiological pH conditions, and they were rather unstable at alkaline conditions and temperature above 25 °C. Among the four dimers tested, B-type (E)C dimer was the least stable, while A-type (E)C dimer was the most stable. In general, B-type dimers showed less stability than A-type ones. The higher the degree of galloylation and the more the hydroxyl groups in the molecular, the less stable the proanthocyanidins dimers.







Similar content being viewed by others
References
Luximon-Ramma A, Bahorun T, Soobrattee MA, Aruoma OI (2002) Antioxidant activities of phenolic, proanthocyanidin, and flavonoid components in extracts of Cassia fistula. J Agric Food Chem 50(18):5042–5047
Akhtar S, Meeran SM, Katiyar N, Katiyar SK (2009) Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting insulin-like growth factor binding protein-3, tumor cell proliferation, and angiogenic factors. Clin Cancer Res 15(3):821–831
Engelbrecht A-M, Mattheyse M, Ellis B, Loos B, Thomas M, Smith R, Peters S, Smith C, Myburgh K (2007) Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer Lett 258(1):144–153
Nandakumar V, Singh T, Katiyar SK (2008) Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett 269(2):378–387
Wu X, Gu L, Prior RL, McKay S (2004) Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J Agric Food Chem 52(26):7846–7856
Wang R, Zhou W, Jiang X (2008) Mathematical modeling of the stability of green tea catechin epigallocatechin gallate (EGCG) during bread baking. J Food Eng 87(4):505–513
Hertog MG, Feskens EJ, Kromhout D, Hertog M, Hollman P, Hertog M, Katan M (1993) Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 342(8878):1007–1011
Li N, Taylor LS, Mauer LJ (2011) Degradation kinetics of catechins in green tea powder: effects of temperature and relative humidity. J Agric Food Chem 59(11):6082–6090
Friedman M, Levin CE, Lee SU, Kozukue N (2009) Stability of green tea catechins in commercial tea leaves during storage for 6 months. J Food Sci 74(2):H47–H51
Zhu QY, Holt RR, Lazarus SA, Ensunsa JL, Hammerstone JF, Schmitz HH, Keen CL (2002) Stability of the flavan-3-ols epicatechin and catechin and related dimeric procyanidins derived from cocoa. J Agric Food Chem 50(6):1700–1705
Lu W-C, Huang W-T, Kumaran A, Ho C-T, Hwang LS (2011) Transformation of proanthocyanidin A2 to its isomers under different physiological pH conditions and common cell culture medium. J Agric Food Chem 59(11):6214–6220
Xiao J-S, Liu L, Wu H, Xie B-J, Yang E-N, Sun Z-D (2008) Rapid preparation of procyanidins B2 and C1 from Granny Smith apples by using low pressure column chromatography and identification of their oligomeric procyanidins. J Agric Food Chem 56(6):2096–2101
Dong X-Q, Zou B, Zhang Y, Ge ZZ, Du J, Li C-M (2013) Preparation of A-type proanthocyanidin dimers from peanut skins and persimmon pulp and comparison of the antioxidant activity of A-type and B-type dimers. Fitoterapia 91:128–139
Wang R, Zhou W, Wen RAH (2006) Kinetic study of the thermal stability of tea catechins in aqueous systems using a microwave reactor. J Agric Food Chem 54(16):5924–5932
Kirca A, Özkan M, Cemeroglu B (2003) Thermal stability of black carrot anthocyanins in blond orange juice. J Food Qual 26(5):361–366
Beecher GR (2003) Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr 133(10):3248S–3254S
Ree T (2001) Tannins: classification and definition. Nat Prod Rep 18(6):641–649
Zhu QY, Zhang A, Tsang D, Huang Y, Chen Z-Y (1997) Stability of green tea catechins. J Agric Food Chem 45(12):4624–4628
Wang R, Zhou W, Jiang X (2008) Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. J Agric Food Chem 56(8):2694–2701
Liazid A, Palma M, Brigui J, Barroso CG (2007) Investigation on phenolic compounds stability during microwave-assisted extraction. J Chromatogr A 1140(1):29–34
Kosińska A, Xie Y, Diering S, Héritier J, Andlauer W (2012) Stability of phenolic compounds isolated from cocoa, green tea and strawberries in Hank’s balanced salt solution under cell culture conditions. Pol J Food Nutr Sci 62(2):91–96
Guo Q, Zhao B, Shen S, Hou J, Hu J, Xin W (1999) ESR study on the structure–antioxidant activity relationship of tea catechins and their epimers. Biochim et Biophys Acta Gen Subj 1427(1):13–23
Balde A, Pieters L, Wray V, Kolodziej H, Berghe D, Claeys M, Vlietinck A (1991) Dimeric and trimeric proanthocyanidins possessing a doubly linked structure from Pavetta owariensis. Phytochemistry 30(12):4129–4135
Malien-Aubert C, Dangles O, Amiot MJ (2002) Influence of procyanidins on the color stability of oenin solutions. J Agric Food Chem 50(11):3299–3305
Kosinska A, Xie Y, Diering S, Heritier J, Andlauer W (2012) Stability of phenolic compounds isolated from cocoa, green tea and strawberries in Hank’s balanced salt solution under cell culture conditions. Pol J Food Nutr Sci 62(2):91–96
Gariboldi E, Mascetti D, Galli G, Caballion P, Bosisio E (1998) LC-UV-electrospray-MS-MS mass spectrometry analysis of plant constituents inhibiting xanthine oxidase. Pharm Res 15(6):936–943
Rodrigues CM, Rinaldo D, dos Santos LC, Montoro P, Piacente S, Pizza C, Hiruma-Lima CA, Brito ARM, Vilegas W (2007) Metabolic fingerprinting using direct flow injection electrospray ionization tandem mass spectrometry for the characterization of proanthocyanidins from the barks of Hancornia speciosa. Rapid Commun Mass Spectrom 21(12):1907–1914
Spencer JPE, Chaudry F, Pannala AS, Srai SK, Debnam E, Rice-Evans C (2000) Decomposition of cocoa procyanidins in the gastric milieu. Biochem Biophys Res Commun 272(1):236–241
Sang SM, Lee MJ, Hou Z, Ho CT, Yang CS (2005) Stability of tea polyphenol (-)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J Agric Food Chem 53(24):9478–9484
Cherviakovsky EM, Bolibrukh DA, Baranovsky AV, Vlasova TM, Kurchenko VP, Gilep AA, Usanov SA (2006) Oxidative modification of quercetin by hemeproteins. Biochem Biophys Res Commun 342(2):459–464
Aura AM, Martin-Lopez P, O’Leary KA, Williamson G, Oksman-Caldentey KM, Poutanen K, Santos-Buelga C (2005) In vitro metabolism of anthocyanins by human gut microflora. Eur J Nutr 44(3):133–142
Fleschhut J, Kratzer F, Rechkemmer G, Kulling SE (2006) Stability and biotransformation of various dietary anthocyanins in vitro. Eur J Nutr 45(1):7–18
Yoshino K, Suzuki M, Sasaki K, Miyase T, Sano M (1999) Formation of antioxidants from (-)-epigallocatechin gallate in mild alkaline fluids, such as authentic intestinal juice and mouse plasma. J Nutr Biochem 10(4):223–229
Hong J, Lu H, Meng XF, Ryu JH, Hara Y, Yang CS (2002) Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res 62(24):7241–7246
Liu H, Zou T, Gao J-M, Gu L (2013) Depolymerization of cranberry procyanidins using (+)-catechin, (-)-epicatechin, and (-)-epigallocatechin gallate as chain breakers. Food Chem 141(1):488–494
Talcott ST, Howard LR (1999) Phenolic autoxidation is responsible for color degradation in processed carrot puree. J Agric Food Chem 47(5):2109–2115
Bermudez-Soto MJ, Tomas-Barberan FA, Garcia-Conesa MT (2007) Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem 102(3):865–874
Ou KQ, Gu LW (2014) Absorption and metabolism of proanthocyanidins. J Funct Foods 7:43–53
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31271833), the Chinese Ministry Program for New Century Excellent Talents in University (NCET-12-0865), Special Fund for Agro-scientific Research in the Public Interest (No. 201203047), and Fundamental Research Funds for the Central Universities (2013PY022).
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Z., Wei, Lh., Ge, Zz. et al. Comparison of the degradation kinetics of A-type and B-type proanthocyanidins dimers as a function of pH and temperature. Eur Food Res Technol 240, 707–717 (2015). https://doi.org/10.1007/s00217-014-2375-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2375-9