Abstract
The objective of this study was to analyse selected brands of fermented (red and black) and unfermented (white and green) teas for free radical content with the use of electron paramagnetic resonance (EPR) spectroscopy and for contents of flavan-3-ols by means of high-performance liquid chromatography. Analyses were also conducted for the polyphenolic profile of infusions of the analysed teas (with the Folin–Ciocalteu’s method) and their antioxidant activity (in reaction with a DPPH radical) at three brewing times (5, 10 and 15 min). The obtained results showed the possibility of using rapid spectroscopic method EPR to evaluate the oxidative changes in tea leaves caused by enzymatic fermentation. The number of free radicals in teas was negatively correlated with contents of flavan-3-ols, (−)-EGCG in particular. The main signals observed in EPR spectra of teas were attributed to semiquinone radicals; however, also signals attributed to carbohydrate radicals were detected. Regarding unfermented teas, it was ascertained that teas with the highest content of flavan-3-ols, (−)-EGCG in particular, were characterised by the lowest content of semiquinone radicals and a high content of carbohydrate radicals. The group of fermented teas demonstrated to contain mainly semiquinone radicals. The total phenolic content and antioxidant activity of the tea infusions were strongly diversified depending on the kind and brand of tea as well as on the extraction time. The predominating flavan-3-ol in the analysed teas was epigallocatechin gallate (−)-EGCG, the content of which was additionally highly correlated with the antioxidant activity of the tea infusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tea, owing to its cherished sensorial values and specific properties, is one of the most frequently consumed beverages around the globe. This has become the key reason behind interest in health-promoting properties of a tea infusion. Recent studies have shown that a tea infusion may affect reduction in the risk of cardiovascular diseases [1, 2] and some forms of cancer development [3], as well as to be implicated in arterial blood pressure decrease and body mass reduction [4]. It has also been shown to exhibit anti-inflammatory, antibacterial, antiviral, neuroprotective and anticoagulating properties [2]. The health-promoting properties of a tea infusion are, to a great extent, determined by the presence in tea leaves of compounds polyphenolic in character that possesses strong antioxidant properties. Other bioactive compounds occurring in tea leaves include alkaloids (e.g. caffeine), amino acids, proteins, carbohydrates, pigments and lipids [5].
The polyphenolic profile of tea leaves is diversified as affected by: geographical location, growing conditions (soil, climate, agricultural practices, fertilisers) and varieties of tea [6]. The major group of phenolic compounds occurring in tea leaves are flavan-3-ols (also referred to as catechins), which constitute approximately 20–30 % by weight of dry matter of tea leaves [7]. The most significant flavan-3-ols occurring naturally in tea leaves include (−)-epigallocatechin gallate ((−)-EGCG), (−)-epigallocatechin ((−)-EGC), (−)-epicatechin gallate ((−)-ECG), (−)-epicatechin ((−)-EC), (+)-gallocatechin ((+)-GC) and (+)-catechin ((+)-C) [8]. Those substances, while migrating to an infusion is imparting it strong antioxidant (health-promoting) properties [9], yet excessive contents of catechins and non-hydrolysing tannins in a tea infusion may yield negative effects on its sensory attributes and colour parameters. Out of the other phenolics present in tea leaves worthy of mention are quercetin, kaempferol and myricetin, occurring in the form of glycosides, theogallin and phenolic acids, including: gallic, p-coumaric and chlorogenic acids [10]. Generally, due to the production method, teas may be divided into: unfermented teas (white and green), semi-fermented teas of Oolong and Paochong type (red), and fermented teas [11]. Unlike white and green teas, the production of fermented teas is based on the fermentation process during which flavan-3-ols present in leaves are oxidised in the presence of an enzyme (polyphenolic oxidase) to ortho-quinones, which in turn are being condensed to theaflavins and thearubigins [12]. These compounds are responsible for the bitter taste of black tea infusion and its dark colour.
Electron paramagnetic resonance (EPR) spectroscopy was successfully used for early detection of oxidative changes in dried food products during storage as well as for evaluation of the protection of food by different antioxidants [13]. However, there is little information related to EPR spectroscopic features of radical species in tea samples. Moreover, literature lacks data reporting attempts of detection of oxidative changes in tea leaves in terms of EPR characteristics. Thus, the quantitative EPR analysis of both fermented and unfermented teas may be an useful method for evaluate the oxidative changes in tea leaves caused by enzymatic fermentation or storage. This information seems to be of key importance owing to the destructive effect of reactive oxygen species on food products. In contrary to black and green teas, white teas are relatively expensive and rarely consumed (apart from the Asian continent). Thus, despite of a number of data concerning the comparison between fermented and unfermented teas in terms of phenolic content and antioxidant activity, there is little information regarding unfermented white teas [14], as well as the influence of brewing time on the mentioned above parameters of tea infusions. This information also seems to be very important for consumers owing to health-promoting and antioxidant properties of phenolic constituents of tea infusions (e.g. flavan-3-ols, flavonoids, theaflavins and thearubigins).
Bearing this in mind, the aim of the reported study was:
-
(a)
to determine the types and number of free radicals in tea leaves using EPR as well as to determine the phenolic profile (flavan-3-ols content) of selected fermented (black and red) and unfermented (white and green) teas of various brands using the high-performance liquid chromatography (HPLC) method
-
(b)
to determine the effect of brewing time of fermented (red and black) and unfermented (white and green) teas on the content of phenolic content in the infusions and its antioxidant activity measured in the reaction with a DPPH radical.
Materials and methods
Reagents
The acetic acid (99.5 %), chloroform, ethyl acetate, methanol, ortho-phosphoric acid (85 %) and sodium carbonate were of analytical grade and supplied by Chempur (Piekary Śląskie, Poland). The acetonitrile (HPLC) grade was purchased from POCh (Gliwice, Poland). The 2,2-diphenyl-1-picrylhydrazyl (DPPH), (−)-epigallocatechin-3-gallate, (−)-epigallocatechin, (−)-epicatechin, (+)-catechin, Folin–Ciocalteu reagent and gallic acid were supplied from Sigma-Aldrich (Steinheim, Germany). The (−)-epicatechin-3-gallate was purchased from Supelco (Bellefonte, USA). The 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) (97 %) was purchased by Aldrich (Steinheim, Germany).
Samples
The experimental materials were 12 teas of four different brands: Bio-Active, Lipton, Teekanne and Yunnan, including: unfermented teas (white and green) of Bio-Active, Lipton, Teekanne and Yunnan brands; fermented teas (black) of Lipton, Teekanne and Yunnan brands; and fermented tea (red) of Bio-Active brand. The analysed teas were purchased in hypermarkets in the city of Krakow, Poland.
Extraction and chromatographic analysis of flavan-3-ols (catechins) in tea samples
A reported procedure [15] was adapted to determinate the flavan-3-ols content in tea samples. In summary, 1 g of commercial green tea was poured with 20 mL of deionised water and heated in a water bath at a temp. of 80 °C for 15 min in the atmosphere of argon to prevent oxidation by atmospheric oxygen. The resultant infusion was collected, and the residues were poured with another portion of water and heated in a water bath under conditions as above. The whole procedure was repeated 3 times. The resultant infusions, having been cooled to a room temperature, were purified by threefold extraction with 20 mL of chloroform in order to remove pigments and methylxanthines. After discarding the chloroform fraction, phenolic compounds (flavan-3-ols) were extracted from the residues 4 times with ethyl acetate each time using 30 mL of the solvent. Fractions of ethyl acetate were collected and evaporated to dryness in a vacuum evaporator in the atmosphere of argon. The residues after evaporation were dissolved in 10 mL of a acetonitrile/water mixture (1:1, v/v). Sample prepared in such way was subjected to a chromatographic analysis (HPLC). For each sample, the extraction procedure was conducted in 2 replications.
The qualitative and quantitative analysis of phenolic compounds (flavan-3-ols) in the teas examined was conducted with the use of HPLC with a reversed-phase (ODS) column based on the method developed by the reported procedure [15]. Before the exact chromatographic analysis, all samples were filtered through Millex-LCR (PTFE) filters with a pore diameter of 0.45 μm. The analyses were conducted using an L-7000 LaChrom liquid chromatograph (Merck-Hitachi, Tokyo, Japan). Out of the phenolic compounds isolated from the samples, analyses were conducted for the following substances: (+)-catechin, (−)-epicatechin, (−)-epigallocatechin, (−)-epigallocatechin gallate and (−)-epicatechin gallate. They were identified with a L-7420 LaChrom UV/Vis detector (Merck-Hitachi, Tokyo, Japan) at a wavelength of λ = 280 nm. The chromatographic separation was carried out on a reversed-phase ODS column (Thermo Scientific, USA: 25 mm × 0.4 cm, particle size 5 μm) at a temperature of 30 °C, in a gradient mode with two phases: phase A—a mixture of acetonitrile, ethyl acetate and 0.1 % ortho-phosphoric acid, (8.5:2:89.5, v/v/v) and phase B—a mixture of acetonitrile and water (1:1, v/v), at eluent flow rate of 1 mL/min. The chromatographic analysis of the samples was conducted for 30 min as follows: the first 10 min—isocratic flow of phase A, 10–20 min—linear gradient through increasing phase B contribution to 20 %, followed by the stable composition of solvents (80 % of phase A and 20 % of phase B) maintained till the 30th minute of chromatographic analysis. The qualitative analysis of flavan-3-ols was conducted by comparing their retention times with those of standards. In turn, the quantitative analysis was carried out by comparing areas of individual peaks on chromatograms with those of calibration curves plotted for particular standards. The calibration curves of the analysed flavan-3-ols were plotted separately for each standard at 4 different concentrations (0.125–1.0 mg/mL) in 3 replications. The chromatographic analysis of each tea sample was conducted in two replications.
Analysis of tea samples by EPR method
Electron paramagnetic resonance (EPR) measurements were performed for solid, untreated tea sample. The samples of 0.05–0.08 g were introduced to EPR quartz tubes, and EPR spectra were recorded using the X-band Bruker Elexsys 500 spectrometer (Karlsruhe, Germany) with 100 kHz field modulation at a room temperature and microwave power of 3.0 mW, in the range of 5 mT. 2,2-diphenyl-1-picrylhydrazyl (DPPH) was used as a g-factor standard. It was applied also as a reference sample (with the number of spins equal to 1.0 × 1015/g) to estimate the amount of radicals.
Electron paramagnetic resonance (EPR) parameters such as values of g-factor, the constants of hyperfine splitting (A), character of lines, their peak-to-peak line width (ΔB pp) and relative intensities of the particular signals of the spectrum were found by the simulation procedure using the program SIM 32 [16]. The fitting of theoretical spectrum to the detected one was performed by the nonlinear least squares method. The theoretical spectrum was the sum of particular signals, with appropriate contributions. The accuracy of determination of EPR parameters was: ±0.0005 for g-factor and ±0.1 mT for parameter A, whereas the accuracy of the radical number calculation was ±0.5 × 1015.
Preparation of tea infusions
The samples (2 g) of the analysed teas were weighed and brewed with 100 mL of distilled water. Fermented teas (black teas and red Bio-Active tea) were brewed at a temperature of 95 °C, whereas unfermented teas (white and green ones) were brewed at 75–80 °C, respectively. Three tea brewing times were applied, that is, 5, 10 and 15 min.
Determination of phenolic content in tea infusions
The total phenolic content (TPC) in infusions of the investigated teas was determined using the Folin–Ciocalteu reagent following the reported procedure [17]. In summary, 2.5 mL of Folin–Ciocalteu reagent (0.2 N) was added to 0.5 mL of tea infusions (diluted with distilled water at the ratio of 1:10, v/v). After 3 min of sample incubation, 2 mL of a 7.5 % Na2CO3 solution was added to the sample. The resulting sample was incubated for 2 h, and then its absorbance was measured at λ = 765 nm against a blank sample (prepared by the addition of first 2.5 mL of Folin–Ciocalteu reagent (0.2 N) and then 2 mL of Na2CO3 solution to 0.5 mL of water) using a UV/Vis V-530 spectrophotometer (Jasco, Tokyo, Japan). The measurements were performed in triplicate. The total content of phenolics was expressed in mg of gallic acid per 1 L of infusion, based on a calibration curve. The latter was plotted for standard solutions of gallic acid used in concentration range of 0–200 mg per 1 L according to the above described procedure.
Determination of antioxidant activity of tea infusions
The antioxidant activity of tea infusions was assayed using a DPPH radical using a method following the reported procedure [18]. In summary, a hundred microlitres of tea infusions [diluted with methanol at the ratio of 1:10, (v/v)] was mixed with 3.9 mL of methanolic solution of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) (25 mg per 1 L) and left for 30 min. Then an absorbance of the mixture was measured in UV/Vis V-530 spectrophotometer (Jasco, Tokyo, Japan) at wavelength at 515 nm against methanol as a blank sample. The measurements were performed in triplicate. The antioxidant activity of the infusions was expressed as trolox equivalent antioxidant capacity (TEAC) in mM of Trolox per 1 L of infusion, using a calibration curve plotted for Trolox methanolic solutions in the concentration range of 0–1.0 mM according to the above described procedure.
Results and discussion
Flavan-3-ols content in tea samples
In this research study, we considered the importance of understanding the role of enzymatic fermentation in the genesis of free radicals generated in tea leaves. The chromatographic analysis of both fermented and unfermented teas enabled to evaluate the content of flavan-3-ols that play a key role in the generation of radical forms of phenolic compounds during enzymatic fermentation.
In the investigated samples of teas (fermented and unfermented ones), quantitative analyses were carried out for the following flavan-3-ols: (−)-epigallocatechin gallate (EGCG), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), (−)-epicatechin (EC) and (+)-catechin (C). The results achieved demonstrated that contents of tea flavan-3-ols differed both between fermented and unfermented teas and among the brands (Table 1). Fermented teas, in particular red Bio-Active tea, were characterised by significantly lower contents of all analysed flavan-3-ols in comparison with the unfermented teas.
A comparison of properties of white and green tea infusions was addressed in a research [19] in which analyses were carried out for the polyphenolic profile of infusions obtained from white to green teas at different brewing times (5, 15 and 30 min) and with various extractants. This research demonstrated that, irrespective of the brewing time, the infusion of green tea was a richer source of flavan-3-ols (e.g. (−)-EGC, (−)-EGCG, (−)-GCG and (−)-ECG) than the white tea infusion; however, extraction of polyphenols from leaves of white tea proceeded considerably slower than in the case of green tea, and extraction yield was strictly depended on the extractant’s composition.
Analysis of free radicals content in teas using EPR
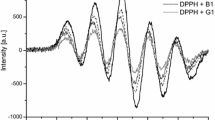
Electron paramagnetic resonance (EPR) spectra of the analysed teas displayed many similarities. Their intensities were found to depend on tea kind and appeared to be the highest in black teas the lowest in green teas (Table 2). The simulation procedure of spectra enabled discriminating 2 or 3 signals of the Gaussian character, depending on kind of tea (Fig. 1). The main signal, with the highest percentage contribution to the spectra, was signal I with parameters similar for all teas (Table 2), whereas signal II exhibited g-factor and ΔB pp width depending on tea kind. The lowest g-factor of this signal was noted for green teas, whereas the highest for black and red tea (Table 2). Widths of signals II were changing from the value of ΔB pp = 0.4–0.7 mT determined for unfermented teas to the value of ΔB pp = 1.2 mT noted for black Yunnan and Lipton teas. Simulation of spectra of two teas, white Bio-Active and black Teekanne, revealed the presence of signal III instead of signal II. Signal III displayed a different characteristics than the signals I and II. It exhibited g-factor in the range 2.0032–2.0034 and hyperfine structure (Table 2). In the spectra of all green and white teas and in the spectra of black Yunnan and Teekanne teas, the simulation required an additional signal IV with a higher value of g-factor, that is, g = 2.0048–2.0057, compared to the other signals (Table 2).
The experimental (exp) and simulated (sim) EPR spectra of different kinds of teas, registered at 293 K, at 3 mW, in the range 5 mT and their particular signal components (I, II, III, IV) used to simulation of the spectra: a Teekane black, b Lipton black, c Lipton green, d Bio-Active green, e Yunnan white I, II—signals of semiquinone radicals, III—carbohydrate radical localised at C(1) atom of glucose, IV—carbohydrate radical localised at C(1) atom of glucose unit in oligosaccharide structure created after dehydration
The observed signals I and II were single lines with EPR parameters being typical for a group of stable semiquinone radicals occurring frequently in plant material [20–25]. The kind of semiquinone radical could be identified on the basis of the hyperfine structure resulting from interactions between magnetic moments of unpaired electron localised at oxygen and nuclear spins of hydrogen atoms. However, in our study, it was impossible to ascribe the signals to define semiquinone structures, since the values of hyperfine splittings characteristic for such species were small [26] and could not be observed in the X-band spectra, because of hyperfine lines overlapping. Therefore, it could be only assumed that the process of semiquinone radical generation resulted from the equilibrium settled between quinone/semiquinone forms. The certain confirmation of such approach was the character of changes of signal II g-factor, which was significantly lower in unfermented teas, the green teas in particular. As demonstrated in a research [20], values of g-factors of semiquinone radicals depended on external conditions the plant material was exposed to and under oxidising conditions the value of g-factor was increasing from 2.0031 to 2.0038. Such a dependency was true for both unfermented and fermented teas. The later possessed radicals exhibiting signal II with higher values of g-factors, which was likely to be due to oxidation reactions occurring during fermentation processes.
In the case of black Teekanne tea and white Bio-Active tea, tangibly marked hyperfine structure of signal III pointed to a different origin of this signal than that of signals I and II. The values of its HFS splitting were significantly higher than those observed for signals of semiquinone radicals [26]; hence, we ascribed tentatively signal III to carbohydrate radical. The formation of such centre could occur under the influence of solar, mechanical, irradiation or thermal energy and was observed in living plants, as well as in other biological materials subjected to γ-irradiation, heating or grinding [27–31]. The radicals were formed via removal of hydrogen atom from carbon of carbohydrate molecules, probably mono or disaccharides [31–33]. Assuming the radical formation at C(1) of glucose, magnetic moment of an unpaired electron localised at this carbon atom could interact with the magnetic moment of the nucleus of the hydrogen atom (I = 1/2) at C(2), leading to the appearance of two lines of hyperfine structure.
The signal IV exhibited a significantly higher intensity in unfermented white and green teas, which might indicate that it was linked with the paramagnetic centre which decayed in the course of the fermentation process. A relatively high value of its g-factor could suggest that the signal originated from a carbohydrate radical, localised probably in polysaccharides structure [27, 28, 30, 34, 35]. The absence of HF lines pointed to the lack of interactions between magnetic moments of an unpaired electron and hydrogen nuclei, which was probably caused by the removal of hydrogen atoms from polysaccharide molecule, for example upon dehydration process, which occurred during drying. Assuming localisation of radical at C(1) of glucose molecule, a unit building polysaccharide molecules, the dehydration occurring via the removal of the hydrogen atom at C(2) and OH group situated at C(3) led to the creation of the double bond between C(2) and C(3) and simultaneous vanishing of HFS of signal [36]. Lower content of the carbohydrate radical in black teas than in unfermented ones was probably caused by carbohydrates degradation occurring during fermentation process. The presence of stable carbohydrate radicals in plant material subjected to drying was confirmed in literature [22].
The main signals observed in EPR spectra of teas were attributed to semiquinone radicals which concentration was determined by the equilibrium between oxidised form of quinone and reduced form of hydroquinone. The quantitative analysis of phenolic compounds in the investigated teas allowed stating that this equilibrium was strongly shifted towards the reduced form, mainly in the unfermented teas. This was fully corroborated by the quantitative analysis of EPR spectra of the teas. The lowest content of semiquinone radicals was found in green and white teas, particularly in green Yunnan and Bio-Active teas and in white Lipton tea, which were characterised by exceptionally high content of flavan-3-ols, (−)-EGCE in particular. The process of fermentation shifted the quinone–hydroquinone equilibrium towards the oxidised form, which resulted in an increased content of semiquinone radical in the fermented black teas and in the red tea. It was accompanied by a drastic decrease in flavan-3-ols content, especially in black Yunnan and Teekanne teas. In the former, the content of the semiquinone radical was exceptionally high.
The analysis of EPR spectra enabled even more precise characteristics of teas of particular kinds. Among green teas, two groups might be discriminated, that is, the first included Yunnan and Bio-Active teas containing a lower number of radicals, while the second included Teekanne and Lipton teas with ca. twice as high number of radicals. The Bio-Active tea was characterised by a higher percentage content of carbohydrate radicals giving signal IV. The second group, including Teekanne and Lipton teas, displayed a higher content of the semiquinone radical responsible for the signal I (Table 2).
Out of the white teas, Teekanne, Yunnan and Bio-Active were showing similar contents of radicals; however, Bio-Active exhibited different EPR characteristics showing signal III, absent in the spectra of the remaining two. The EPR characteristics of Lipton tea was similar to Teekanne and Yunnan, but the radical content was the lowest among white teas (Table 2).
In comparing both types of unfermented white and green teas, it may be concluded that the teas with the highest contents of flavan-3-ols, (−)-EGCG in particular, were characterised by the lowest content of semiquinone radicals and a high content of the carbohydrate radicals, whereas the teas with lower concentration of flavan-3-ols were characterised by higher contents of semiquinone radicals and lower contents of carbohydrate radicals. The group of fermented teas contained mainly semiquinone radicals. The carbohydrate radicals were occurring in minor contents only in Yunnan and Teekanne teas.
Analysis of correlation between content of flavan-3-ols and free radicals assayed in tea leaves
The chromatographic (HPLC) and spectroscopic (EPR) method used to compare fermented with unfermented teas showed the degree of oxidative changes in tea leaves due to enzymatic fermentation. The increase in a number of semiquinone radicals along with decrease in flavan-3-ols content was observed when comparing the fermented teas with unfermented ones. Results achieved in this study demonstrated statistically significant (α = 0.05) negative correlations (Table 3) between the content of individual flavan-3-ols (Table 1) and the content of free radicals in black, green and white teas (Table 2). The fermented red Bio-Active tea that strongly differed in flavan-3-ols content from the other teas (Table 1) was not included in the correlation. The highest value of negative correlation coefficient (R = −0.791) between measured parameters was noted for (−)-EGCG (Table 3). The values of the negative correlation coefficients noted for individual flavan-3-ols were then successively decreasing in the following order: (−)-EGCG > (−)-EC > (−)-ECG > (−)-EGC > (+)-C, however, the correlation between the content of (+)-catechin and the numbers of free radicals in the analysed teas was no longer statistically significant (α = 0.05). The highest value of negative correlation noted for (−)-EGCG (Table 3) is probably due to its highest reactivity as hydrogen or electron donating agent among the analysed flavan-3-ols which resulted in generation a higher amount of radical form of semiquinone in tea leaves caused by oxidation in comparison with other flavan-3-ols. The highest ability of (−)-EGCG for oxidation is due to the presence in this molecule two aromatic rings in the form so-called gallyl and galloyl substituent, each possessing three hydroxyl groups. The degree of hydroxylation of the aromatic ring in a phenol molecule, as well as the structure of phenolic compounds plays a key role in electron and hydrogen donating activity of these compounds [27]. On contrary, the lower values of correlation coefficients, in comparison with (−)-EGCG, were observed for (−)-EGC and (−)-ECG (R = −0.595 and R = −0.662, respectively) (Table 3), each possessing only one aromatic ring in the form of gallyl substituent for the former and galloyl for the latter. Additionally, a lower when compared to (−)-EGCG, but still significant (α = 0.05) correlation coefficient (R = −0.7) (Table 3) was observed for (−)-epicatechin that possesses nor gallyl neither galloyl substituent. What is more, the high negative correlations between flavan-3-ols content and number of free radicals was observed for both the groups of unfermented teas (white and green ones) (Table 3). Thus, the process of generation of semiquinone radicals in tea leaves could take place not only during enzymatic fermentation stage but also may occurs during some stages of production of unfermented teas (e.g. heating or drying) or during storage.
Content of phenolic compounds in tea infusions determined with the Folin–Ciocalteu method
Results of the reaction of tea infusions with the Folin–Ciocalteu’s reagent demonstrated that the TPC in the analysed infusions was strongly diversified depending on the kind and brand of tea as well as on the extraction time. In the case of the unfermented teas, depending on tea brand and brewing time, the TPC value ranged from 299.2 to 1646.2 mg/L infusion for white teas and from 1,135 to 1,608 mg/L infusion for green teas, respectively (Table 4). The infusions of green teas of Yunnan, Bio-Active and Teekanne brands were characterised by higher TPC values, compared to the white teas of respective brands. Only in the case of white Lipton tea (with the highest content of (−)-EGCG among all tea samples examined) was its infusion characterised by a higher TPC value compared to the green tea of this brand. The higher value of TPC in the infusion of green Teekanne tea, compared to the white tea of this brand, despite a lower content of catechins determined with the HPLC method (35.10 vs. 51.05 mg/g) (Table 1), might result from the presence of higher quantities of phenolic compounds that were not assayed chromatographically, including, for example, gallic, 3-O-caffeoylquinic (chlorogenic) acid and p-coumaroylquinic acids, as well as flavonoids other than tea catechins. A comparison of properties of white and green tea infusions was addressed in a research [19] in which analyses were carried out for the phenolic profile of infusions obtained from white tea (Pai Mu Tan Superior) and green tea (Long Jing) at different brewing time (5, 15 and 30 min) and with using various solvents (extractants). This research demonstrated that, irrespective of the brewing time, the infusion of green tea was a richer source of both flavan-3-ols and TPC than the white tea infusions. In the case of TPC, our results confirm this thesis only in the case of Bio-Active, Yunnan and Teekanne brands. Moreover, the infusions of white teas of Lipton and Teekanne brands were characterised by a higher content of flavan-3-ols determined chromatographically (Table 1) compared to the green teas of these brands.
The analysis of the effect of extraction time on TPC value in the infusions of unfermented teas revealed that in the case of the white teas, the TPC value in infusions was increasing along with extending extraction time, reaching the maximum after 15 min, except for the infusion of Teekanne tea. For each analysed brand of white tea, statistically significant (α = 0.05) differences were observed in TPC values among all applied extraction times (Table 4). When it comes to the green tea infusions, in the case of Yunnan tea, the TPC value was increasing significantly (α = 0.05) along with extraction time till the 15th minute, whereas in the case of Bio-Active and Teekanne Brand teas, the maximum TPC value was achieved as soon as after 15 min for the former and after 10 min for the latter, while in the case of Lipton tea no statistically significant (α = 0.05) differences were observed in TPC value, irrespective of the extraction time. It was also observed that in the case of the white teas (except of white Lipton brand), the process of extraction of phenolic compounds proceeded considerably slower in comparison with the green teas. The greatest differences in the TPC values, as affected by the extraction time, were recorded between the 5th and 10th minute of extraction, but still those differences were more tangible in the case of the white tea infusions in comparison with the green ones. It is consistent with the previously reported research [19] which also demonstrated a slower extraction process of phenolic compounds for white tea, compared to green tea.
The content of total phenolics in the infusions of fermented teas ranged from 722 to 1,246 mg/L infusion (Table 4). In the case of Lipton and Bio-Active brands, a statistically significant increase (α = 0.05) in TPC was observed along with extending extraction time. Irrespective of the extraction time, the highest content of phenolic compounds was determined for the infusion of black Lipton tea (Table 4) which was the richest in (−)-EGCG (Table 1) out of all fermented teas examined.
Antioxidant activity of tea infusions determined in the reaction with a DPPH radical
Values of the antioxidant activity of the investigated infusions of fermented and unfermented teas were expressed as TEAC in mmol Trolox per 1 L of extract (Table 5). The infusions of unfermented teas (white and green ones) were characterised by significantly higher values of the antioxidant activity, compared to the infusions of fermented teas. Likewise in the case of TPC (Table 4), the infusions of green teas of Yunnan, Bio-Active and Teekanne brands exhibited higher values of the antioxidant activity than infusions of the white teas of the same brands. Out of the fermented teas, the highest values of the antioxidant activity were determined for the infusions of Lipton tea, which appeared to be the richest source of (−)-EGCG among all brands of fermented teas. High and statistically significant (α = 0.05) correlations were noted between TPC in tea infusions, determined with the reaction with Folin–Ciocalteu reagent, and the antioxidant activity of tea infusions assayed in the reaction with a DPPH radical, in the case of all applied brewing times (5, 10 and 15 min), R = 0.963, 0.958 and 0.961, respectively.
When comparing contents of individual flavan-3-ols determined with the HPLC method in the investigated teas (Table 1) with the antioxidant activity of their infusions obtained after the longest time of brewing (15 min) (Table 5), the highest and significant (α = 0.05) value of a correlation coefficient out of all flavan-3-ols examined was noted for (−)-EGCG (R = 0.798). This is consistent with literature data [37] referring to the antiradical activity of flavanols determined in the reaction with a DPPH radical, which confirms the highest activity of (−)-EGCG against a DPPH radical out of all flavan-3-ols occurring naturally in tea leaves.
Conclusions
Analysis of the obtained results showed the possibility of using the spectroscopic method (EPR) to evaluate the degree of oxidation occurring during processing of tea leaves (e.g. enzymatic fermentation). The applicability of chromatographic methods (HPLC) for evaluation the influence of enzymatic fermentation of tea leaves on the degree of oxidation (e.g. decreasing in flavonoids and phenolic acids content, increasing in theaflavins content) has been widely used and known. The proposed spectroscopic method (EPR) enables to identify the particular types of radical species generated in studied material during processing or storage. Moreover, the analysis of correlation between a number of particular types of free radical species and the content of bioactive compounds in studied material may be helpful in better understanding of radical mechanism of oxidation processes occurring during food processing and storage. This information seems to be significantly important owing to destructive influence of reactive oxygen species on food products both processing and storage.
The quantitative EPR analyse of the teas under study enabled to state the type and number of free radicals in teas under study. The EPR spectra of dry tea leaves proved that the main and most intensive signals were originated from semiquinone radical species. However, also signals attributed to the stable carbohydrate radicals were detected. An increasing content of semiquinone radicals in the fermented teas was caused by enzymatic fermentation of tea leaves that shifted the quinone–hydroquinone equilibrium towards the more oxidised form. When comparing the both groups of unfermented teas (green and white), it was ascertained that the teas with the highest contents of flavan-3-ols, (−)-EGCG in particular, were characterised by the lowest content of semiquinone radicals and a high content of the carbohydrate radicals. The group of fermented teas under study demonstrated to contain mainly semiquinone radicals. The obtained results revealed statistically significant trend between the number of free radicals and contents of individual flavan-3-ols (in particular, (−)-EGCG). TPC and antioxidant activity of the analysed tea infusions were strongly diversified depending on the kind and brand of tea, as well as on extraction time. The tea infusions of unfermented teas showed higher values of both TPC and antioxidant activity than those of fermented ones. In the case of most of the white teas under study the process of extraction of phenolic compounds proceeded considerably slower than in the green teas. The predominating flavan-3-ol in the analysed teas was epigallocatechin gallate (−)-EGCG, the content of which was significantly and highly correlated with the antioxidant activity of tea infusions evaluated in the reaction with a DPPH radical. Also, the high and significant correlations were confirmed between the TPC and the antioxidant activity of tea infusions, in the case of all applied brewing times.
References
Hertog MGL, Feskens EJM, Hollman PCM, Katan MB, Kromhout D (1993) Lancet 342:1007–1011
Kuńyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y (2006) JAMA 296:1255–1265
Nichenametla SN, Taruscio TG, Barney DL, Exon JH (2006) Crit Rev Food Sci Nutr 46:161–183
Sueoka N, Suganuma M, Sueoka E, Okabe S, Matsuyama S, Imai K, Nakachi K, Fujiki H (2001) Ann NY Acad Sci 928:274–280
Chen Y-S, Liu B-L, Chang B-L (2010) J Biosci Bioeng 109(6):557–563
Cabrera C, Artacho R, Giménez R (2006) J Am Coll Nutr 25:79–99
Wang H, Provan GJ, Helliwell K (2000) Trends Food Sci Technol 11:152–160
Nishitani E, Sagesaka YM (2004) J Food Compos Anal 17:675–685
Gadow A, Joubert E, Hansmann CF (1997) Food Chem 60:73–77
Novak I, Šeruga M, Komorsky-Lovrić Ŝ (2010) Food Chem 122:1283–1289
Bancirova M (2010) Food Res Int 43:1379–1382
Rawel HM, Kulling SE (2007) J Verbr Lebensm 2:399–406
Nissen LR, Huynh-Ba T, Petersen MA, Bertelsen G, Skibsted LH (2002) Food Chem 79:387–394
Zhao Y, Chen P, Lin L, Harnly JM, Yu L, Li Z (2011) Food Chem 126:1269–1277
Baptista Jose AB, da P Tavares JF, Carvalho RCB (1999) J Food Comp Anal 12: 273–287
Spałek T, Pietrzyk P, Sojka Z (2005) J Chem Inf Model 45:18–29
Waterhouse AL (2002) In: Current protocols in food analytical chemistry. Wiley, New York, p I1.1.1
Sánchez-Moreno C, Larrauri JA, Rupérez P, Saura-Calixto F (1999) J Food Sci Agric 79: 1301–1304
Rusak G, Komes D, Likić S, Horžić D, Kovać M (2008) Food Chem 110:852–858
Jezierski A, Czechowski F, Jerzykiewicz M, Golonka I, Drozd J, Bylinka E, Chen Y, Seaward MRD (2002) Spectrochim Acta A 58:1293–1300
Leprince O, Atherton NM, Deltour R, Hendry GAF (1994) J Plant Physiol 104:1333–1339
Łabanowska M, Filek M, Kościelniak J, Kurdziel M, Kuliś E, Hartikainen H (2012) J Plant Physiol 169:275–284
Polat M, Korkmaz M (2008) Food Chem 107:956–961
Atherton NM, Hendry GAF, Mobius K, Rohrer T, Torring J (1993) Free Rad Res Commun 19:297–301
Suhaj M, Rácová J, Polovka M, Brezová V (2006) Food Chem 97:696–704
Pedersen JA (2002) Spectrochim Acta A 58:1257–1270
Kuzuya K, Yamauchi Y, Kondo S (1999) J Phys Chem B 103:8051–8059
Polovka M, Brezová V, Staško A, Mazur M, Suhaj M, Šimko P (2006) Radiat Phys Chem 75:309–321
Korkmaz M, Polat M (2001) Radiat Phys Chem 62:411–421
Łabanowska M, Bidzińska E, Pietrzyk S, Juszczak L, Fortuna T, Błoniarczyk K (2011) Carbohydr Polym 85:775–785
Madden KP, Bernhard WA (1982) J Phys Chem 86:4033–4036
Adhikary A, Becker D, Collins S, Koppen J, Sevilla MD (2006) Nucleic Acid Res 34:1501–1511
Yamauchi M, Sugito M, Kuzuya M (1999) Chem Pharm Bull 47:273–278
Łabanowska M, Bidzińska E, Dyrek K, Fortuna T, Pietrzyk S, Rożnowski J, Socha RP (2008) Starch-Staerke 60:134–145
Yamaoki R, Kimura S, Ohta M (2010) Radiat Phys Chem 79(4):417–423
Łabanowska M, Wesełucha-Birczyńska A, Kurdziel M, Sepioło K (2013) Carbohydr Polym 91:339–347
Almajano MP, Carbó R, Jiménez JAL, Gordon MH (2008) Food Chem 108:55–63
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Socha, R., Bączkowicz, M., Fortuna, T. et al. Determination of free radicals and flavan-3-ols content in fermented and unfermented teas and properties of their infusions. Eur Food Res Technol 237, 167–177 (2013). https://doi.org/10.1007/s00217-013-1975-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-013-1975-0