Abstract
Chlorogenic acid, the ester of caffeic acid with quinic acid, is a natural phenolic compound found in all higher plants. It is potentially useful in pharmaceuticals, foodstuffs, feed additives, and cosmetics due to recently discovered biomedical activity of this compound. This finding caused new interest in the properties of chlorogenic acid in its isomers and in its natural occurrence. It has been found that as many as fourteen compounds (chlorogenic acid derivatives and its reaction products with water) can be formed from 5-O-caffeoylquinic acid by heating its water solution at different pH. Four of them, two hydroxylated 3-O-caffeoylquinic acid derivatives and two hydroxylated 4-O-caffeoylquinic acid derivatives, have been not reported yet. The amount of each formed component depends on the heating time and pH. The transformation product can be mistakenly treated as a new component, not found before in the examined plant, or can be a cause of erroneous quantitative estimations of plant composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chlorogenic acids (CGA) are a large family of esters formed between one and more residues of phenolic acid (usually caffeic, ferulic, or p-coumaric) and quinic acid. These compounds are widely spread in plants and many food products, diet supplements, and beverages [1, 2].
5-O-Caffeoylquinic (5-CQA)—being the ester of caffeic acid with quinic acid, naturally occurring in the highest concentration—is the most studied compound of the chlorogenic acid family. Potential uses of 5-CQA are suggested in pharmaceuticals, dietary (foodstuffs, food additives), and cosmetics due to its recently discovered biomedical activity [3–9]. This finding caused new interest in 5-CQA properties, its isomers, and its natural occurrence.
As previously reported [10], the heating of 5-CQA water solution in the temperature range of 100–200 °C causes chlorogenic acid isomerization and transformation. It was found that as many as nine compounds (chlorogenic acid derivatives and its reaction products with water) can be formed from 5-O-caffeoylquinic acid by heating of its water solution [10]. The amount of each formed component depends on the heating time and temperature. As results from [11], 5-CQA in alkaline water solution undergoes isomerization and transformation even at room temperature. According to [11], two isomers of chlorogenic acid—3-O-caffeoylquinic acid (3-CQA) and 4-O-caffeoylquinic acid (4-CQA)—and a methyl derivative of caffeic acid are formed in 5-CQA aqueous solution containing tetramethylammonium hydroxide. The question asked in the present study is about the influence of pH on 5-CQA thermal stability during its heating in buffered water solution. The presented results may be valuable for researchers examining plant materials in which chlorogenic acid derivatives formed during extraction can be mistakenly treated as the natural components of the examined plants.
Experimental section
Reagents
Acetonitrile (HPLC), sodium phosphate, and phosphoric acid (both of analytical grade) were purchased from the Polish Chemical Plant—POCh (Gliwice, Poland); formic acid was from Sigma–Aldrich (Seelze, Germany); chlorogenic acid was from Loba-Chemie Austranal Praparate (Austria). Water was purified on the Milli-Q system from Millipore (Millipore, Bedford, MA, USA).
Methods
The investigations into pH influence on the 5-O-caffeoylquinic acid transformation process were performed by heating 5-O-caffeoylquinic acid buffered water solution under reflux. The glass equipment composed of a boiling flask (100 mL) and a small condenser was used for this purpose. Phosphate buffers of following pH were applied during experiments: 4.0, 5.0, 6.0, 7.0, 8.0, and 9.0. The heated 5-O-caffeoylquinic acid solutions contained 10 mg of 5-O-caffeoylquinic acid in 50 mL of buffer. The acid portions were inserted to the already boiling buffer. The solvents were heated for 10 min or 1 or 3 or 5 h.
HPLC measurements
Chromatographic measurements were performed using LC/ESI/IT/MS from Finnigan (LCQ Adventage Max) equipped with the ion-trap mass spectrometric system (ThermoElectron Corporation, San Jose, CA) and a diode array detector from Finningan (Surveyer PDA Plus Detector). The column used was a 100 × 4.6 mm i.d., 3 (m, Gemini C18 (Phenomenex, Torrance, CA, USA). Chromatographic separation was performed using gradient elution. Mobile phase A was 25 mM formic acid in water; mobile phase B was 25 mM formic acid in acetonitrile. The gradient program started at 5% B increasing to 35% for 30 min, next 35% B to 100% B for 5 min, followed by isocratic elution followed (100% B) by 5 min. The total run time was 40 min at mobile phase flow rate 0.4 mL/min.
In the cause of each run, PDA spectra in the range 200–600 nm and MS spectra in the range of m/z 100–1,000 were collected continuously. SIM function was used to better visualize the chromatographic separation and to remove the signal connected with buffer components; 0–8 min (191 m/z), 8–13 min (371 m/z), 13–15 min (153 m/z), 15–20, 5 min (353 m/z), 20,5–23 min (179 m/z), 23–40 min (353 m/z).
The column effluent was ionized by electrospray (ESI). The ESI needle potential was 4.5 kV in the negative ionization mode. To identify the chlorogenic acid isomers and the chlorogenic acid transformation products, the functions of secondary (MSn) ion fragmentation were applied. The collision energy for each examined compounds was chosen individually.
To identify hydroxylated 5-O-caffeoylquinic acid derivatives in examined samples, LC/ESI/TOF/MS analysis was additionally performed. LC/ESI/TOF/MS analysis was carried out on the Agilent (Agilent Technologies, Palo Alto, CA, USA) liquid chromatography system. MS analysis was performed on the orthogonal TOF/MS equipped with an electrospray interface (Agilent Technologies, Santa Clara, CA, USA). Negative mode using full scan mode in TOF/MS analysis was applied, and the mass range was set 100–400 Da. Chromatographic separation was performed using the same chromatographic column and the same gradient elution as described for LC/ESI/IT/MS analysis.
Due to the lack of hydroxylated derivatives of 5-CQA, 4-CQA and 3-CQA, 1-O-caffeoylquinic acid (1-CQA), 3-CQA, 4-CQA and of cis-5-O-caffeoylquinic standards (cis-5-CQA), the amounts of these compounds were calculated by relating their chromatographic responses to the calibration curve for 5-CQA. The amounts of cis caffeic acid (cis-CA) were estimated using the calibration curve for trans-caffeic acid (CA).
Results and discussion
Investigations into the 5-O-caffeoylquinic acid thermal stability in water buffer were performed by heating a buffered water solution of 5-CQA under reflux. Ten milligram portions of 5-CQA were used in all experiments as one such portion is more or less equivalent to the amount of the compound in a 0.5 g sample of many plants, that is, a plant sample mass typically used in assisted extraction processes such as pressurized liquid extraction (PLE) or microwave-assisted solvent extraction (MASE).
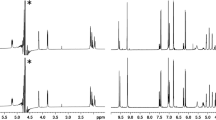
Figure 1 presents the chromatograms of the 5-CQA water solution (Fig. 1a) and of the 5-CQA heated under reflux for 5 h in buffered water solution at pH = 6.0 (Fig. 1b) and at pH = 8.0 (Fig. 1c). The last two samples imitate the chlorogenic acid extract obtained for this compound during its hot water extraction at definite pH. As shown in Fig. 1b and 1c, the 5-CQA water buffer solution contains, besides the parent substance, fourteen additional compounds formed as a result of the 5-CQA transformation and degradation. Eight of them (quinic acid (QA), peak 1; protocatechuic acid (PA), peak 8; trans 1-CQA, peak 9; 3-CQA, peak 10; 4-CQA, peak 12; CA, peak 13; cis-CA, peak 14; and cis-5-CQA, peak 15) were identified and confirmed on the basis of the retention data of their standards and of the PDA, MSn [12, 13], and HRMS data. It is worth mentioning that the absorption maximum for cis-CA and cis-5-CQA occurs at shorter wavelength than for their trans form [14].
Chromatograms of 5-CQA water solution (a) and of 5-CQA heated under reflux for 5 h in buffered water solution at pH = 6.0 (b) and at pH = 8 (c). Peak numbers correspond to compound numbers in Fig. 2. The sequence of diastereoisomeric components in 2/3 pair or 4/5 or 6/7 should be treated tentatively as we were not able to differentiate R or S component in given pair
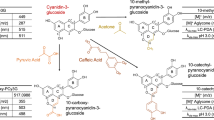
For better clarity, the names of all the identified compounds, their peaks and structure numbers, shortcuts, and chemical structures are collected in Table 1 and graphically in Fig. 2. It was assumed that there was no epimerization in quinic moiety during 5-CQA transformation process. The compound names used in the table and the text are in agreement with IUPAC nomenclature of cyclitols [15]. The MSn and HRMS data for the mentioned compounds are collected in Table 2 and 3.
Molecular structures of 5-O-caffeoylquinic and of its transformation products: 1 quinic acid, 2 (1S,3R,4R,5R)-3-[3-(3,4-dihydroxyphenyl)-3R-hydroxypropanoyl] 1,4,5-trihydroxycyclohexanecarboxylic acid, 3 (1S,3R,4R,5R)-3-[3-(3,4 dihydroxyphenyl)-3S-hydroxypropanoyl]-1,4,5-trihydroxycyclohexanecarboxylic acid, 4 (1S,3R,4R,5R)-5-[3-(3,4-dihydroxyphenyl)-3R-hydroxypropanoyl]-1,3,4-trihydroxycyclohexanecarboxylic acid, 5 (1S,3R,4R,5R)-5-[3-(3,4-dihydroxyphenyl)-3S-hydroxypropanoyl]-1,3,4-trihydroxycyclohexanecarboxylic acid, 6 (1S,3R,4R,5R)-4-[3-(3,4-dihydroxyphenyl)-3R-hydroxypropanoyl]-1,3,5-trihydroxycyclohexanecarboxylic acid, 7 (1S,3R,4R,5R)-4-[3-(3,4-dihydroxyphenyl)-3S-hydroxypropanoyl]-1,3,5-trihydroxycyclohexanecarboxylic acid, 8 protocatechuic acid, 9 1-O-caffeoylquinic acid, 10 3-O-caffeoylquinic acid, 11 5-O-caffeoylquinic acid, 12 4-O-caffeoylquinic acid, 13 caffeic acid, 14 cis-caffeic acid, 15 cis-5-O-caffeoylquinic acid
PDA, MSn, and HRMS data indicate that six other compounds (peaks 2–7 in Fig. 1b) can be identified as the following diastereoisomeric pairs of hydroxylated derivatives of 5-CQA, 4-CQA, and 3-CQA:
(1S,3R,4R,5R)-5-[3-(3,4-dihydroxyphenyl)-3R-hydroxypropanoyl]-1,3,4-trihydroxycyclohexanecarboxylic acid (peak 4 in Fig. 1b and structure 4 in Fig. 2) and (1S,3R,4R,5R)-5-[3-(3,4-dihydroxyphenyl)-3S-hydroxypropanoyl]-1,3,4-trihydroxycyclohexanecarboxylic acid (peak 5 in Fig. 1b and structure 5 in Fig. 2); (1S,3R,4R,5R)-3-[3-(3,4-dihydroxyphenyl)-3R-hydroxypropanoyl]-1,4,5-trihydroxycyclohexanecarboxylic acid (peak 2 in Fig. 1b and structure 2 in Fig. 2); and (1S,3R,4R,5R)-3-[3-(3,4-dihydroxyphenyl)-3S-hydroxypropanoyl]-1,4,5-trihydroxycyclohexanecarboxylic acid (peak 3 in Fig. 1b and structure 3 in Fig. 2); (1S,3R,4R,5R)-4-[3-(3,4-dihydroxyphenyl)-3R-hydroxypropanoyl]-1,3,5,-trihydroxycyclohexanecarboxylic acid (peak 6 in Fig. 1b and structure 6 in Fig. 2) and (1S,3R,4R,5R)-4-[3-(3,4-dihydroxyphenyl)-3S-hydroxypropanoyl]-1,3,5,-trihydroxycyclohexanecarboxylic acid (peak 7 in Fig. 1b and structure 7 in Fig. 2). As results from [10], the first two hydroxylated chlorogenic acid derivatives (the first diastereoisomeric pair) can be formed even during the heating of 5-CQA in pure water. The molecular weights of the compounds represented by peaks 2–7 are identical within experimental error (Table 2; 3) and exceed molecular weight of the chlorogenic acid by 18 Da, the value corresponding to the molecular weight of water (18.0106 Da). The addition of the water molecule to the double bond in a given CQA isomer should result in the formation of the similar amounts of its two hydroxylated derivatives (diastereoisomers) with similar physicochemical properties. The relations between peak intensities corresponding to the individual pairs of hydroxylated CQA derivatives should be similar to the relations between the peaks for their precursors. As results from the obtained chromatograms, the heights of peaks 2 and 3 are very similar. The same is observed for peaks 4 and 5 or 6 and 7. Moreover, the relation between the heights of peak pairs 2/3:4/5:6/7 is similar as the relation between the heights of peaks 10:11:12 that correspond to 5-CQA, 4-CQA, and 3-CQA, the precursors of hydroxylated CQA derivatives.
The retention times of components in given diastereoisomeric pair are very close, indicating similarity of their physicochemical properties. The introduction of the OH group to any molecule increases its polarity, which results in a shorting of its retention time in the RP chromatographic system. Thus, the retention times of the considered compounds are shorter than the retention times of their precursors, indicating that their polarity is higher than that of CQA isomers.
MSn spectra of individual pair peaks are very similar to the MSn spectra of their precursors. MSn spectra of the components of a given pair are less helpful in differentiating hydroxylated chlorogenic acid derivatives as both derivatives are fragmented to ions of very similar mass and intensity (Table 1).
All the presented evidences support the correctness of the identification. Moreover, the introduction of hydroxyl group in β position to carbonyl group in CQA is in agreement with Michael’s acceptor theory [16].
The influence of pH and heating time on the amount of individual components formed during 5-CQA transformation and degradation is presented in Figs. 3, 4, 5, 6, 7. The presented relationships show:
Influence of pH on the amount of cis-5-CQA (a) and 5-CQA-3R-OH or 5-CQA-3S-OH (b or c)* formed during the 5-CQA heating in buffered water solution for 10 min (dotted line), 1 h (dash-dotted line), 3 h (dashed line), and 5 h (solid line). *We were not able to ascribe the peaks to the specified diastereoisomer
Influence of pH on the amount of 3-CQA-3R-OH or 3-CQA-3S-OH (a or b)* and 4-CQA-3R-OH or 4-CQA-3S-OH (c or d)* formed during the 5-CQA heating in buffered water solution for 10 min (dotted line), 1 h (dash-dotted line), 3 h (dashed line) and 5 h (solid line). *We were not able to ascribe the peaks to the specified diastereoisomer
-
the increase in the QA, CA, and cis-CA amount in 5-CQA solution with the increase in pH (see Fig. 3). These dependences relate to the products of 5-CQA hydrolysis (QA, CA) and to cis-CA (being the CA isomerization product) and prove that pH increase accelerates 5-CQA hydrolysis. Moreover, the increase in heating time of 5-CQA solution causes the increase in the amounts of the mentioned compounds. The amounts of formed QA and CA are similar, which agrees with the reaction stoichiometry of 5-CQA hydrolysis. High concentration of hydrogen ions inhibits the formation process. The comparison of the plots in Figs. 3b, c reveals that the amount of cis-CA strongly depends on the CA concentration and the heating time. At short heating times, below 3 h, cis-CA is not detected;
-
the increase in hydroxylated 5-CQA derivates (5-CQA-3R-OH and 5-CQA-3S-OH) and cis-5-CQA with the decrease in pH (see Fig. 4). This is due to the formation of these compounds from 5-CQA carbocation. Higher concentration of hydrogen ions promotes the formation of the carbocation. The comparison of the amount of 5-CQA-3R-OH, 5-CQA-3S-OH, and cis-5-CQA shows that the transformation of 5-CQA carbocation to hydroxylated 5-CQA derivates is more privileged than its transformation to the cis-5-CQA isomer;
-
the initial increase in the compound amount for 1-CQA, 3-CQA, and 4-CQA isomers, hydroxylated 3-CQA and 4-CQA derivates (3-CQA-3R-OH, 3-CQA-3S-OH, and 4-CQA-3R-OH, 4-CQA-3S-OH, respectively) and of PA followed by the decrease in their amount in the heated 5-CQA solution (see Figs. 5, 6, 7). This shape of the relationships can be explained by the occurrence of two competitive reactions at simultaneous degradation of the initial substrate: the formation of transformation products and their degradation. The plots in Fig. 5 require additional discussion. According to them, the increase in heating time of 5-CQA solution shifts the position of the maximum of the shown relationship toward lower pH. It is especially evident for 1-CQA. Such shift suggests that the formation of the isomers at lower pH prevails over their degradation. Higher pH favors the degradation of the formed CQA isomers. Figure 5 additionally shows that the formation of 4-CQA predominates over that of 3-CQA and much more so over that of 1-CQA. This phenomenon is known from the literature [16] and can be explained by different thermodynamic stability (possibly due to intramolecular hydrogen bond contacts) of these CQA isomers. Moreover, steric hindrance occurs during the formation of 1-CQA. The difference in the thermodynamic stability of CQA isomers is revealed in the amount of hydroxylated 3-CQA and 4-CQA derivates (compare Figs. 5, 6). Hydroxylated 1-CQA derivates were not detected—probably due to their lowest precursor concentration, 1-CQA. Figure 7 illustrates the influence of pH on PA formation during the heating of 5-CQA water solution. QA is the precursor of PA. As results from Fig. 3a, trace amounts of QA are detected in 5-CQA water solution in the pH range from 5 to 6. The amount of PA in this pH range is negligible (Fig. 7). This comparison shows a very rapid transformation of QA into protocatechuic acid in more acidic solutions. This agrees with the literature reporting the catalytic influence of hydrogen ions on the dehydration of hydroxylated and polyhydroxylated compounds [16].
Conclusions
The presented results show that as many as fourteen compounds (chlorogenic acid derivatives and its reaction product with water) can be formed from 5-O-caffeoylquinic acid during the heating of its water solution at different pH. Four of them—two hydroxylated 3-O-caffeoylquinic acid derivatives and two hydroxylated 4-O-caffeoylquinic acid derivatives—have not been reported yet. The amount of each formed component depends on the heating time and pH.
Sample preparation is a crucial step in the chemical analysis of plant material. Liquid extraction is currently applied most frequently as a sample preparation procedure for plant analysis. To increase extraction efficiency, buffering extractants are frequently applied. The type of plant matrix also influences extractant pH, which can differ significantly. The results of this study prove that 5-O-caffeoylquinic acid, during its water extraction, can undergo transformations such as isomerization, esterification, hydrolysis, and the addition of water molecule. The transformation products can be mistakenly treated as new components, not found before in the examined plant. They can also lead to erroneous quantitative estimations of plant composition when some or all components formed from 5-O-caffeoylquinic acid during its water extraction really exist in the examined plant and the 5-O-caffeoylquinic acid transformation process only increases their amount. It is worth noticing that occasionally encountered phenyl-hydracrylic and hydroxyl-phenyl-hydracrylic acids in plant extracts could either be artifacts formed during isolation process of bio-components from biomatrix. This is why presented results are important for researchers investigating plant metabolism and looking for new plant components.
References
Clifford MN, Wu W, Kuhnert N (2006) The chlorogenic acids of Hemerocallis. Food Chem 95:574–578
Clifford MN (1999) Review. Chlorogenic acid and other cinnamates—nature, occurrence and dietary burden. J Sci Food Agric 79:362–372
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54:519–546
Jassim SAA, Naji MA (2003) Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol 95:412–427
Rodriguez de Sotillo D, Hadley M, Wolf-Hall C (1998) Potato peel extract a nonmutagenic antioxidant with potential antimicrobial activity. J Food Sci 63:907–911
Bowles BL, Miller AJ (1994) Caffeic acid activity against Clostridium botulinum spores. J Food Sci 59:905–908
Marinova EM, Toneva A, Yanishlieva N (2009) Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem 114:1498–1502
Paynter NP, Yeh HC, Voutilainen S, Schmidt MI, Heiss G, Folsom AR, Brancati FL, Kao WH (2006) Coffee and sweetened beverage consumption and the risk of type 2 diabetes mellitus. Am J Epidemiol 164:1075–1084
Prabhakar PK, Doble M (2009) Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine 16:1119–1126
Dawidowicz AL, Typek R (2010) Thermal stability of 5-O-caffeoylquinic acid in aqueous solutions at different heating conditions. J Agric Food Chem 58:12578–12584
Clifford MN, Kellard B, Birch GG (1989) Characterisation of chlorogenic acids by simultaneous isomerisation and transesterification with tetramethylammonium hydroxide. Food Chem 33:115–123
Markowicz-Bastos DH, Saldanha LA, Catharino RR, Sawya A, Cunha IBS, Carvalho PO, Eberlin MN (2007) Phenolic antioxidants identified by ESI-MS from yerba mate (Ilex paraguariensis) and green tea (Camelia sinesis) extractas. Molecules 12:423–432
Clifford MN, Johnston KL, Knight S, Kuhnert N (2003) Hierachical scheme for LC-MSn identification of chlorogenic acids. J Agric Food Chem 51:2900–2911
Dranik LI, Shubert TA (1970) Cis-trans isomerization of esters of hydroxycinnamic acid. Khimiya Prirodnykh Soedinenii 6:264–265
AC IUP (1976) Nomenclature of cyclitols. Biochem J 153:23–31
McMurry J (2007) Organic chemistry, 7th edn. Brooks/Cole-Thomson, Belmont
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Dawidowicz, A.L., Typek, R. The influence of pH on the thermal stability of 5-O-caffeoylquinic acids in aqueous solutions. Eur Food Res Technol 233, 223–232 (2011). https://doi.org/10.1007/s00217-011-1513-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-011-1513-x