Abstract
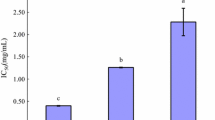
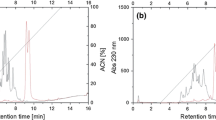
Apricot almond meal was hydrolyzed simultaneously with Neutrase and N120P proteases. The hydrolysate almond peptide (AP) was fractionated into three ranges of molecular weight (AP-I, >5 kDa; AP-II, 1–5 kDa; AP-III, <1 kDa) using an ultrafiltration membrane bioreactor system. The AP-III brought about a high angiotensin-I-converting enzyme (ACE) inhibitory activity with IC50 value of 0.138 mg mL−1 and the content of hydrophobic amino acid of AP-III was 50.08%. Lineweaver–Burk plots suggested that AP-III acts as a non-competitive inhibitor to inhibit ACE. And we evaluated the stability and in vivo antihypertensive activity of AP-III. Multiple dose oral administration (100 mg kg−1 body weight (BW), 400, 800 mg kg−1 BW) to spontaneously hypertensive rats led to a significant decrease in blood pressure for AP-III. Additionally, the gel filtration and RP-HPLC were used to separate ACE inhibitory peptides from the hydrolysate. The results suggested that the peptide derived from apricot almond protein may have potential applications as functional food.



Similar content being viewed by others
References
Jung WK, Mendis E, Je JY, Park PJ, Son BW, Kim HC, Choi YK, Kim SK (2006) Angiotensin I converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem 94:26–32
Fujita H, Yokoyama K, Yoshikawa M (2000) Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J Food Sci 65:564–569
Rencland R, Lithell H (1994) Angiotensin-converting enzyme in human skeletal muscle. A simple in vitro assay of activity in needle biopsy specimens. Scand J Clin Lab Invest 54:105–111
Ondetti MA, Rubin B, Cushman DW (1982) Enzyme of the rennin angiotensin system and their inhibitors. Annu Rev Biochem 51:283–308
Kim SK, Byun HG, Park PJ, Shahidi F (2001) Angiotensin I converting enzyme inhibitory peptides purified from bovine skin gelatin hydrolysate. J Agric Food Chem 49:2992–2997
Miguel M, Contreras MM, Recio I, Aleixandre A (2009) ACE inhibitory and antihypertensive properties of a bovine casein hydrolysate. Food Chem 12:211–214
Je JY, Qian ZJ, Byun HG, Kim SK (2007) Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Proc Biochem 42:840–846
Kuba M, Tana C, Tawata S, Yasuda M (2005) Production of angiotensin I-converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase. Proc Biochem 40:2191–2196
Miyoshi S, Ishikawa H, Kaneko T, Fukui F, Tanaka H, Maruyama S (1991) Structures and activity of Angiotensin-converting enzyme inhibitors in a α-zien hydrolysate. Agric Biol Chem 55:1313–1318
Matsui T, Li CH, Osajima Y (1999) Preparation and characterization novel bioactive peptides responsible for angiotensin I converting enzyme inhibition from wheat germ. J Pept Sci 5:289–297
Hsu FL, Lin YM, Lee MH, Lin CL, Hou WC (2002) Both Dioscorin the tuber storage protein of yam (Dioscorea alata c v, Tainong No 1), and its peptic hydrolysates exhibited angiotensin converting enzyme inhibitory activities. J Agric Food Chem 50:6109–6113
Yust MM, Pedroche J, Giron-Calle J, Alaiz M, Millan F, Vioque J (2003) Production of ACE inhibitory peptides by digestion of Chickpea legumin with Alcalase. Food Chem 81:363–369
Ahrens S, Venkatachalam M, Mistry AM, Lapsley K, Sathe SK (2005) Almond (Prunus dulcis L.) protein quality. Plant Foods Hum Nutr 60:123–128
Cherif A, Sebei K, Boukhchina S, Kallel H, Belkacemi K, Arul J (2004) Kernel fatty acid and triacylglycerol composition for three almond cultivars during maturation. J Am Oil Chem Soc 81:901–905
Abd El-Aal MH, Hamza MA, Rahma EH (1986) In vitro digestibility, physicochemical and functional properties of apricot kernel proteins. Food Chem 19:197–211
Abd E-A, Hamz MA, Sze-Tao KWC, Sathe SK (2000) Functional properties and in vitro digestibility of almond (Prunus dulcis L.) protein isolate. Food Chem 69:153–160
Tiwari RS, Venkatachalam M, Sharma GM, Su M, Roux KH, Sathe SK (2010) Effect of food matrix on amandin, almond (Prunus dulcis L.) major protein, immunorecognition and recovery. Food Sci Technol 43:675–683
Sheng X, Wang Z, Xu S (2008) Study on functional properties and structure of sugary almond protein. Sci Technol Food Industry 29:133–136
Wang S, Wen Z, Li H (2008) Analysis of the nutritional components and contents of sweet apricot kernel. Beijing Agric 9:13–15
Cushman DW, Cheung HS (1971) Spectrophotometric assay and properties of the Angiotensin converting enzymes of rabbit lung. Biochem Pharmacol 20:1637–1648
Lahogue V, Rehel K, Taupin L, Haras D, Allaume P (2010) A HPLC-UV method for the determination of angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem 118:870–875
Vercruysse L, Camp JV, Morel N, Roug P, Herregods G, Smagghe G (2010) Ala-Val-Phe and Val-Phe: ACE inhibitory peptides derived from insect protein with antihypertensive activity in spontaneously hypertensive rats. Peptides 31:482–488
Zhao Y, Li B, Liu Z, Dong S, Zhao X, Zeng M (2007) Antihypertensive effect and purification of an ACE inhibitory peptide from sea cucumber gelatin hydrolysate. Proc Biochem 42:1586–1591
Jiang Z, Tian B, Brodkorb A, Huo G (2010) Production, analysis and in vivo evaluation of novel angiotensin-I-converting enzyme inhibitory peptides from bovine casein. Food Chem 123:779–786
Lee DH, Kim JH, Park JS, Choi YJ, Lee JS (2004) Isolation and characterization of a novel angiotensin I-converting enzyme inhibitory peptide derived from the edible mushroom Tricholoma giganteum. Peptides 25:621–627
Wu J, Ding X (2002) Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res Int 35:367–375
Quirós A, Ramos M, Muguerza B, Delgado MA, Miguel M, Aleixandre A (2007) Identification of novel antihypertensive peptides in milk fermented with Enterococus faecalis. Int Dairy J 17:33–41
Cheung HS, Wang FL, Ondetti MA, Sabo EF, Cushman DW (1980) Binding of peptide substrates and inhibitors of angiotensin converting enzyme. J Biol Chem 255(2):401–407
Suetsuna K, Nakano T (2000) Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida). J Nutr Biochem 11:450–454
Jung WK, Mendis E, Je JY, Park PJ, Son BW, Kim HC, Choi YK, Kim SK (2006) Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem 94:26–32
Maeno M, Yamamoto N, Takano T (1996) Identification of an antihypertensive peptide from casein hydrolysate produced by a proteinase from Lactobacillus helveticus CP790. J Dairy Sci 79:1316–1321
Matsufuji H, Matsui T, Seki E, Osajima K, Nakashima M, Osajima Y (1994) Angiotensin I-converting enzyme inhibitory peptides in an alkaline protease hydrolyzed derived from sardine muscle. Biochem Biotechnol 58:2244–2245
Nakagomi K, Yamada R, Ebisu H, Sadakane Y, Akizawa T, Tanimura T (2000) Isolation of acein-2, a novel angiotensin-I-converting enzyme inhibitory peptide derived from a tryptic hydrolysate if human plasma. FEBS Lett 467:235–238
Erdmann K, Cheung BWY, Schröder H (2008) The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J Nutr Biochem 19:643–654
Acknowledgments
This research was funded by Major Special Project of the Xinjiang Uygur Autonomous Region (200831108).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Tian, J. & Wang, Q. ACE inhibitory and antihypertensive properties of apricot almond meal hydrolysate. Eur Food Res Technol 232, 549–556 (2011). https://doi.org/10.1007/s00217-010-1411-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1411-7