Abstract
A trypsin-chymotrypsin inhibitor, designated Limenin, with both antifungal and antibacterial activity, and exhibiting a molecular mass of 18.0 kDa in SDS–polyacrylamide gel electrophoresis, was isolated from the large lima bean (Phaseolus limensis) legumes by a combination of extraction, ammonium sulfate precipitation, ion exchange chromatography on SP-Toyopearl and high performance liquid chromatography (HPLC) on Mono S. The isoelectric point was estimated to be 7.6 by isoelectric focusing. The 15 N-terminal amino acid sequences were determined to be DFVIDNEGNPLENGG, demonstrating some resemblance to those other protease inhibitors and inhibitor precursors from leguminous plants. It exerted potent antifungal action toward Botrytis cinerea, Alternaria alternata(Fr.) Keissl, and Pythium aphanidermatum. It showed antiproliferative activity toward tumor cells including human liver hepatoma cells Bel-7402 and neuroblastoma cells SHSY5Y. However, it had no effect on bacteria Staphylococcus aureus and Salmonella.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that fungal infection results in serious damage to crops throughout the world. Antifungal proteins have captured the attention of a number of researchers on account of their tremendous potential in protecting crops from invading fungi and thus important economic implication. Antifungal proteins may contribute to both defense against predators such as insects [1] as well as pathogens such as fungi [2, 3]. It is well identified that there is a spectacular diversity of antifungal proteins produced by the leguminous plants. Some of these proteins and peptides have been classified into different groups based on their structure and/or functions [4]. Sometimes, a combination of antifungal proteins is found in a single species of bean. For instance, proteins and peptides such as lysozyme, non-specific lipid transfer protein, and protease inhibitor were isolated from mung bean, and all have fungi inhibition activity [2, 3, 5].
Protease inhibitors have drawn the attention of a number of investigators due to their potential significance. HIV protease inhibitors and SARS coronavirus proteinase inhibitors may be used to fight against HIV and SARS virus, respectively, [6]. Plant protease inhibitors may be involved in the regulation of programed cell death in plants. They show insecticidal and antifungal activities and also can cause inhalant allergies and food allergies [3, 7]. One of the common types of protease inhibitors is trypsin inhibitors which have been isolated from animal tissues and also from plant tissues [7, 8]. There are several types of plant trypsin inhibitors. Kunitz-type trypsin inhibitors have a molecular mass of about 20 kDa, a low cysteine content and a single reactive site while Bowman–Birk trypsin inhibitors are approximately 8 kDa in size and possess a high cysteine content and two reactive sites [9, 10]. The conformation in Kunitz-type trypsin inhibitors is mainly β sheet with a small amount of regular sheet. The insecticidal activity of Kunitz-type trypsin inhibitors has been demonstrated using transgenic plants [8]. Kunitz-type trypsin inhibitors also inhibit other enzymes such as chymotrypsin, a-amylase and human plasmin, and block the conversion of prothrombin to thrombin [7]. Soy bean (Glycine max) produced both Bowman–Birk and Kunitz-type trypsin inhibitors [11]. The formation, degradation, and gene expression of Kunitz-type trypsin inhibitor in the soy bean have also been reported [7]. The seeds of bitter gourds, sponge gourds, wax gourds, and Momordica cochinchinensis produce squash-type trypsin inhibitors with a molecular mass of about 3 kDa [12].
As one of leguminous plants, the large lima bean is also very popular in human being’s daily life. It usually serves as a healthy food because of the abundant proteins and peptides it produces. Proteins such as a chitinase [13], a peroxidase [14], and a defense peptide [15] have previously been reported from large lima bean, the three bioactive substances all exhibiting antifungal activity to varying degrees. We present herein a novel trypsin-chymotrypsin inhibitor, designated Limenin, from the large lima bean (Phaseolus limensis) legume, which exerts both antifungal activity against a range of fungal species as well as antiproliferative activity on tumor cells. Its N-terminal amino acid sequence bears resemblance to those of protease inhibitors from other leguminous plants but has not previously been reported from Phaseolus limensis.
Materials and methods
Materials
Dried large lima bean seeds were purchased from a local supermarket and ranked as the first market class. Affi-gel blue gel, SP-Toyopearl, and Mono S were purchased from BIO-RAD Co. (USA), TOSOH Co. (Japan), and GE Healthcare (Sweden), respectively. Bel-7402, human liver hepatoma cell line, and SHSY5Y, neuroblastoma cells were purchased from the American Type Culture Collection (ATCC, USA). DMEM, F12 medium, RPMI-1640 medium and fetal bovine serum were from Gibco (Grand Island, NY, USA). Standard proteins for molecular mass determination were purchased from Gibco-BRL (Life Tech., USA). All chemicals were of the highest purity available.
Sample preparation
Exactly 150 g of large lima seeds were washed, soaked in distilled water for 12 h and homogenized in 0.1 M Tris–HCl buffer (pH 7.2). The homogenate was centrifuged at 10,000g for 20 min at 4 °C. The supernatant was designated as the crude extract for the further investigations.
Isolation and purification
Ammonium sulfate precipitation
The crude sample was first fractionated by ammonium sulfate precipitation, in which the crude solution was treated with ammonium sulfate to 20% saturation. The resulting supernatant was then adjusted to 85% saturated ammonium sulfate. After centrifugation at 10,000g for 20 min, the supernatant was discarded while the precipitate was collected and dissolved in 100 mL of 0.01 M Tris–HCl buffer (pH 7.2).
Affinity chromatography
The solution of ammonium sulfate precipitate was dialyzed against 0.01 M Tris–HCl buffer (pH 7.2) with several changes, and then applied to an open column of an Affi–gel blue gel column (ϕ2.5 cm × 10 cm) previously equilibrated with the starting buffer, 0.01 M Tris–HCl buffer (pH 7.2). The flow rate was 0.5 mL/min, 10 min/tube, and the eluate was monitored at 280 nm. Following removal of a large amount of unadsorbed proteins, the column was eluted with a linear gradient of NaCl (0–0.5 M) in the same buffer.
Cation-exchange chromatography
The adsorbed fraction demonstrating antifungal activity was pooled, dialyzed against 2,000 mL of 0.01 M Tris–HCl buffer, pH 7.2 at 4 °C for 24 h, and subsequently chromatographed on a column of SP-Toyopearl (ϕ1.2 × 9.5 cm) which had been equilibrated with 0.01 M Tris–HCl buffer (pH 7.2). After elution of a sizeable quantity of unadsorbed materials, the column was eluted with a gradient of NaCl (0–0.5 M) in the same buffer. The flow rate was 1 ml/min, and the absorbances of all fractions were monitored at 280 nm. The first adsorbed fraction (marked as SP1) from SP-Toyopearl column was pooled, dialyzed against 2,000 mL of 0.01 M Tris–HCl buffer, pH 7.2 at 4 °C overnight with several changes, then SP1 was further purified by high performance liquid chromatography(HPLC) on a Mono S column (ϕ 0.6 × 5 cm) in 10 mM Tris–HCl buffer (pH 7.2). The second absorbance peak SPS2 represented purified trypsin-chymotrypsin inhibitor.
Capillary liquid chromatography
The purified Limenin was chromatographed on a C18 capillary reversed phase HPLC column from Sigma -Aldrich Co. (St. Louis, MO, USA) with an analyzer (Applied Biosystems Model ABI 140D, Perkin Elmer Co., MA, USA).
Characterization of the purified Limenin
SDS–PAGE
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) (12.5%T, 4%C) was performed according to the method [16]. Gels were stained in 0.1% (w/v) Coomassie blue-30% (v/v) methanol-10% (v/v) acetic acid in water. The destaining solution was 30% (v/v) methanol-10% (v/v) acetic acid in water.
Isoelectric focusing electrophoresis
The isoelectric focusing (IEF)-PAGE was performed using a 2-Dimensional Electrophoresis and Data Analysis System (InvestigatorTM 5000, Tokyo, Japan). The PhastGel IEF for standard proteins was bought from BIO-RAD Company, USA, covering the pH range 3–10.
Protein determination
Protein concentrations were determined by the method [17] using bovine serum albumin as a standard.
N-terminal amino acid sequence analysis
The N-terminal amino acid sequence of the purified Limenin was performed by Edman degradation using a protein sequencer (Applied Biosystems Model 476A, Perkin Elmer Co. MA, USA). Phenylthiohydantoin derivatives were separated and identified by capillary reversed phase HPLC in a C18 column with an analyzer.
Measurement of trypsin-chymotrypsin inhibitory activity
Ten portions containing the inhibitor of 0, 25, 50, 75, 100, 150, 200, 250, 350 and 450 μg was incubated, respectively, with 25 μg trypsin or chymotrypsin in 100 μL of 50 mM Tris–HCl buffer (pH 8.0) containing 200 mM CaCl2 for 5 min at 25 °C. The reaction was terminated by adding 1 ml of cold 5% trichloroacetic acid after 15-min incubation. Residual trypsin or chymotrypsin activity was determined by adding 300 μLof 1% casein substrate at 25 °C. The reaction mixtures were centrifuged for 20 min at 8,000g, and then the absorbance of the supernatant was determined at 280 nm.
Assay for antibacterial activity
The assay for antibacterial activity toward Staphylococcus aureus, Salmonella was conducted using sterile petri dishes (100 × 15 mm) containing 10 mL LB agar (1.5% agar). Three milliters of warm nutrient agar (0.7%) containing the bacteria were poured into the plates. A sterile blank paper disk (0.625 cm in diameter) was placed on the agar. Then a solution containing 50 μM Limenin in 20 mM Tris–HCl buffer (pH 7.2) was introduced to a disk. The plates were incubated at 37 °C for 12–20 h. A transparent ring around the paper disk was used to identify whether the sample has antibacterial activity.
Assay for antifungal activity
The assay for antifungal activity was executed using 100 × 15 mm petri plates containing 10 mL of potato dextrose agar. Around and at a distance of 1 cm away from the central disk (0.625 cm in diameter) were placed sterile blank paper disks of the same size. An aliquot (8 μL containing 50 μg or 180 μg) of Limenin in 20 mM Tris–HCl buffer (pH 7.2) was introduced to a disk. The plates were incubated at 23 °C for 72 h until mycelial growth from the central disk had enveloped peripheral disks containing the control (buffer) and had produced crescents of inhibition around disks containing samples with antifungal activity. The fungal species included Botrytis cinerea, Alternaria alternata(Fr.) Keissl, Pythium aphanidermatum, Mycosphaerella arachidicola, Fusarium oxysporum, and Sclerotium rolfsii.
For a quantitative assay for antifungal activity, Pythium aphanidermatum was taken as an example, three doses (containing 5, 15, and 30 μM) of the Limenin in 20 mM Tris–HCl buffer pH 7.2 were added separately to three aliquots each containing 4 mL potato dextrose agar at 45 °C, mixed rapidly, and poured into 3 separate small petri dishes. After the agar cooled down, the same small amount of mycelia was inoculated onto each plate. Buffer only without antifungal protein served as a negative control. After incubation at 27 °C for 72 h, the area of the mycelial colony was measured, and the inhibition of fungal growth was expressed as IC 50 , which represents the protein concentration required for 50% growth inhibition.
Assay for antiproliferative activity
The antiproliferative activity of Limenin on tumor cells was carried out by testing its inhibition of the growth of the human liver hepatoma cells of Bel-7402 and the neuroblastoma cells of SHSY5Y. Soy bean protein isolate was used as control for excluding the general effect of protein. The cells of Bel-7402 were cultured in RPMI-1640 medium supplemented with 10% (v/v) fetal calf serum; the cells of SHSY5Y were cultured in DMEM/F12 (1:1) medium supplemented with 10% (v/v) fetal bovine serum, in a humidified atmosphere of 5% CO2 at 37 °C. The cells (3 × 105 cells/150 μL/well) were seeded into a 96-well culture plate, and a series of solutions containing Limenin in 150 μL medium were added. After incubation of the cells at 37 °C in a humidified atmosphere of 5% CO2 for 24 h, the cells were then harvested and dyed with MTT. The absorbance of the samples at 590 nm was determined using a microtiter plate (ELISA) reader and was directly correlated to the level of its anti-tumor activity. The inhibitory activity of the Limenin was calculated as the percent inhibition compared to a control without the sample.
To evaluate the detail for its antiproliferative effect, the inhibition of growth in tumor cell lines of SHSY5Y by purified sample was observed under a reversed microscopic observation. After 24 h of growth in the presence of 75 μM Limenin, morphology of the cell lines were observed with the × 100 magnification.
Statistical analyses
All experiments were conducted in triplicate. All data were presented as means (standard deviations, SDs) of three independent experiments. Statistical analysis was done using Student’s t test. A value of P < 0.05 was considered statistically significant.
Results and discussion
Purification of the Limenin
The solution of the ammonium sulfate precipitate was applied to an open column of Affi-gel blue gel. Following removal of a large amount of unadsorbed proteins(B0), the adsorbed fraction (B1) exhibiting antifungal activity was desorbed from the Affi-gel column with a linear NaCl concentration gradient (Fig. 1a). The active peak was pooled, dialyzed against 0.01 M Tris–HCl buffer, pH 7.2 for 24 h, and chromatography on a SP-Toyopearl column was carried out. The active peak SP1 was pooled and subsequently dialyzed (Fig. 1b). After that Mono S column on HPLC was carried out (Fig. 1c). The second peak, which is designated SPS2, displayed both inhibition activity and antifungal activity. The purified Limenin was shown by capillary reversed phase high performance liquid chromatography (Fig. 1d) to be of high purity. Its yields at each purification step are presented in Table 1. From 150-g lima bean legumes via the Mono S column, 6.5 mg of a purified trypsin inhibitor was obtained.
a Fractionation of a solution of the (NH4)2SO4 precipitate extract on a Affi-gel blue gel column equilibrated with the binding buffer (0.01 M Tris–HCl buffer, pH 7.2). The gel was washed with the binding buffer and eluted with a linear gradient of 0–0.5 M NaCl in the same buffer. b Elution profile of fraction from the Affi-gel blue gel column. The adsorbed fraction from the Affi-gel blue gel column was pooled, dialyzed, and then applied to a SP-Toyopearl column in 0.01 M Tris–HCl buffer, pH 7.2. The column was then washed with the binding buffer. Adsorbed proteins were eluted with a linear gradient of NaCl from 0 to 0.5 M in the same buffer. c The adsorbed fraction SP1 from SP column was pooled, dialyzed, and applied to high performance liquid chromatography on a Mono S column. Protein elution was carried out with 0.01 M Tris–HCl buffer, pH 7.2. The second absorbance peak SPS2 represented purified trypsin-chymotrypsin inhibitor. d Capillary reversed phase high performance liquid chromatography of the purified Limenin using a C18 column (a.i. means the detection signal)
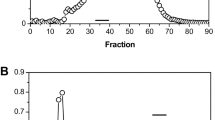
The purified Limenin exhibited a single band on SDS–polyacrylamide gel electrophoresis under both non-reducing and reducing conditions (with or without dithiothreitol), as shown in Fig. 2. This result indicated it was a monomeric protein rather than have multiple subunits from a protein. The molecular weight of the Limenin obtained was estimated to be 18 kDa (Fig. 2). The isoelectric point (pI) of Limenin was determined to be 7.6 according to the results of isoelectric focusing electrophoresis shown in Fig. 3.
SDS-polyacrylamide gel electrophoresis of purified Limenin. From left to right: Lane M was molecular mass standards; lane dithiothreitol- (DTT-) was loaded with 8 μg Limenin under non-reducing conditions (without addition of DTT); Lane DTT + was loaded with 8 μg Limenin under reducing conditions (with DTT added)
The Kunitz-type trypsin inhibitor isolated in the present investigation from large lima bean (Limenin) was very similar to those of previously reported antifungal proteins [4, 6, 12–15, 18–20] It was adsorbed on affinity chromatography on Affi-gel blue gel, and a series of ion chromatography such as SP-Toyopearl and Mono S. In contrast to various molecular masses reported for antifungal proteins and peptides [4, 5, 18, 19, 22, 23], the molecular mass of Limenin is within the range of low molecular masses. Limenin demonstrated a basic isoelectric point, showing the common property with the antifungal proteins reported before [14, 15, 20].
The 15 N-terminal amino acid sequence of the purified lima bean Limenin was determined to be DFVIDNEGNPLENGG. This demonstrated varying resemblance (between 40 and 93%) to those of Kunitz-type trypsin inhibitors from wild soybean, black soybean, yellow soybean, and winged soybean according to the results of a BLAST Search. The N-terminal sequence of the purified trypsin inhibitor Limenin was some homologous to those of Kunitz-type trypsin inhibitors from other leguminous plants (Table 2).
Trypsin and chymotrypsin inhibitory activities
The Limenin inhibited trypsin and chymotrypsin activity as shown in Fig. 4. When the molar ratio of inhibitor to chymotrypsin equaled to 3, the enzyme activity was inhibited 50% of activity. However, the inhibition capacity to trypsin activity was much stronger than that to chymotrypsin, when the molar ratio of the Limenin to chymotrypsin equaled to 10, the residual enzyme activity was hardly calculated (Fig. 4). Limenin, the newly purified protein, inhibited the activity of both trypsin and chymotrypsin, and the potency toward trypsin is more higher, which is similar to its counterpart from black soybean (G. max) and wild soybean [24]. It represents broad bean trypsin inhibitor [8].
Antifungal and antibacterial activity
The antifungal activity of lima bean Limenin against fungal species was illustrated in Fig. 5I–III. It can be seen that the protein showed obvious antifungal activity toward Botrytis cinerea (Fig. 5I), Alternaria alternata(Fr.) Keissl (Fig. 5II), and Pythium aphanidermatum (Fig. 5III). In addition, the IC 50 value of the antifungal activity toward Pythium aphanidermatum was calculated to be 12.3 μM (Fig. 6). Light microscopic photographs showed the hyphal morphological distortion and poor hyphal quantity in those fungi growing in the presence of Limenin when compared with the growth on control medium showing normal hyphal development (data not shown). However, it showed hardly any antifungal activity on fungi Mycosphaerella arachidicola, Fusarium oxysporum, and Sclerotium rolfsii. It had no effect on bacteria Staphylococcus aureus and Salmonella (data not shown).
Trypsin inhibitor Limenin displays a spectrum of activities. It inhibits mycelial growth in a few of fungal species, like some of the previously reported antifungal proteins [3, 5, 18, 19, 22]. Interestingly, a chitinase [13], a peroxidase [14], and a defensin peptide [15] have previously been reported from large lima bean, and all exhibited antifungal effects to varying degrees. It may be reasonable to deduce that a combination of antifungal proteins is present in this variety of lima bean and that they work together to defend against the attacks from invading pathogens such as disease-causing fungi. Like any other leguminous plant, lima legumes have evolved a variety of potent defense mechanisms, including proteins and peptides that have antifungal activity. The existence of proteins with antifungal activity might represent a selective advantage toward a wide range of potential pathogens activity.
Antiproliferative activity on tumor cells
The antiproliferative activity of Limenin to the human liver hepatoma cells of Bel-7402, the neuroblastoma cells of SHSY5Y were calculated as percent inhibition compared to a control without the sample. According to the inhibition results (Fig. 7), the IC 50 value toward Bel-7402 was calculated to be 50 μM, while the IC 50 value toward SHSY5Y was calculated to be 41 μM. Soy bean protein isolate was used as control for excluding the general effect of protein. Not surprisingly, soy bean protein showed no significant antiproliferative activity on the cells mentioned above (data not shown). Reversed microscopic observations on inhibition of growth in tumor cell SHSY5Y by purified Limenin were shown as Fig. 8. Morphological distortion and poor cell quantity was observed after 48 h of purified sample treatment.
Some reported antifungal proteins also demonstrate in addition antibacterial activities [2, 3, 5, 20, 23, 25] and antiproliferative activities on tumor cell lines [3, 15, 20, 21, 23, 24]. The investigation is a significant addition when compared with those of the aforementioned counterparts.
To summize, Limenin is a trypsin-chymotrypsin inhibitor with antiproliferative activity toward cancer cells Bel-7402 and SHSY5Y, antifungal activity toward several fungal species. This investigation is the first that demonstrates an antifungal inhibitor with antiproliferative potency in large lima beans. The versatile biological activities showed by lima bean trypsin-chymotrypsin inhibitor that imply exploitable potentials for the therapeutic potential of this class of antifungal protein.
References
Murdock LL, Huesing JE, Nielsen SS, Prat RC, Shade RE (1990) Phytochem 29:85–89
Wang SY, Ng TB, Chen T, Lin DY, Rao PF, Ye XY (2005) Biochem Biophys Res Commun 327:820–827
Wang SY, Lin J, Ye MY, Ng TB, Rao PF, Ye XY (2006) Peptides 27:3129–3136
Ng TB (2004) Peptides 25:1215–1222
Wang SY, Wu JH, Ng TB, Ye XY, Rao PF (2004) Peptides 25:1235–1242
Ng TB, Huang B, Fong WP, Yeung HW (1997) Life Sci 61:933–949
Birk Y (2003) Berlin, Heidelberg, New York: Springer
Zhao M, Naude RJ, Muramoto K, Oelofsen W (1996) Int J Pept Protein Res 48:174–181
Cavalcanti MDM, Oliva MLV, Fritz H, Jochum M, Mentele R, Sampaio M, Coelho LCBB, Batista IFC, Sampaio CAM (2002) Biochem Biophys Res Commun 291:635–639
Kumar P, Rao AG, Hariharaputran S, Chandra N, Gowda LR (2004) J Biol Chem 279:30425–30432
Deshimaru M, Hanamoto R, Kusano C, Yoshimi S, Terada S (2002) Biosci Biotechnol Biochem 66:1897–1903
Wong RC, Fong WP, Ng TB (2004) Peptides 25:163–169
Wang SY, Zhou J, Shao B, Lu YJ, Rao PF (2008) J Food Sci 73(6):452–457
Wang SY, Gong YS, Zhou J (2009) J Food Sci 74(3):c193–c198
Wang SY, Rao PF, Ye XY (2009) Applied Microbiol Biotech 82:79–86
Laemmli UK, Favre M (1973) J Mol Biol 80:575–599
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) J Biol Chem 193:265–275
Vogelsang R, Barz W (1993) Planta 189:60–69
Vu L, Huyhn QK (1994) Biochem Biophys Res Commun 202:666–672
Wang SY, Shao B, Rao PF, Lee YY, Ye XY (2007) J Agric Food Chem 55:9792–9799
Qureshi A, Colin PL, Faulkner DJ (2000) Tetrahedron 56:3679–3685
Raj PA, Dentino AR (2002) FEMS Microbiol Lett 266:9–18
Mastrolorenzo A, Rusconi S, Scozzafava A, Barbaro G, Supuran CT (2007) Current Medical Chem 14:2734–2748
Lin P, Ng TB (2008) Process Biochem 43:992–998
Flores T, Alape-Giron A, Flores-Diaz M, Flores HE (2002) Plant Physiol 128:1291–1302
Acknowledgments
This research was supported by the Science and Technology Foundation of Fuzhou University, P. R.China (Number: 2009XQ18) and the Excellent Talents Support Program (2010), Fujian Province, P. R.China. The authors are grateful to Dr. XiaoLiang Shi in the College of Agricultural and Life Science at University of Wisconsin, Madison, USA, for valuable assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Rao, P. A leguminous trypsin-chymotrypsin inhibitor Limenin with antifungal activity from Phaseolus limensis . Eur Food Res Technol 231, 331–338 (2010). https://doi.org/10.1007/s00217-010-1285-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1285-8