Abstract
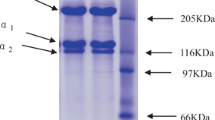
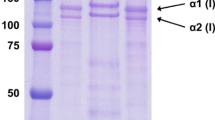
Acid-soluble collagen (ASC) and pepsin-soluble collagen (PSC) from the skin of blacktip shark (Carcharhinus limbatus) were isolated and characterized. The yield of ASC (20.01%) was much higher than that of PSC isolated from the residue of ASC extraction (0.86%). Both collagens had protein as their major constituent with the trace amounts of ash and fat. Based on protein patterns and TOYOPEARL® CM-650M column chromatography, both collagens contained α- and β-chains as their main components and were characterized as type I collagen with the cross-link of α2-chain. Similar peptide maps of both collagens, digested by either V8 protease or lysyl endopeptidase, were observed but they were totally different from those of type I collagen from calf skin hydrolyzed by the same enzyme. Thermal transition temperature (T max) of ASC and PSC were 34.23 and 34.37 °C, respectively. Fourier-transform infrared spectra suggested that both collagens were in triple-helical structure. From zeta potential analysis, isoelectric points (pI) of ASC and PSC were estimated to be 6.78 and 7.02, respectively. Thus, blacktip shark skin may serve as an alternative source of collagen and acid solubilization process could be implemented with ease and high yield.






Similar content being viewed by others
References
Balian G, Bowes JH (1977) In: Ward AG, Courts A (eds) The science and technology of gelatin, Academic Press, London
Sadowska M, Kolodziejska I, Niecikowska C (2003) Food Chem 81:257–262
Regenstein JM, Zhou P (2007) In: Shahidi F (ed) Maximising the value of marine by-products, 1st edn. Woodhead Publishing Limited, Cambridge
Musick JA (2005) In: Musick JA, Bonfil R (eds) Management techniques for elasmobranch fisheries. FAO fisheries technical paper no. 474, FAO, Rome
Bae I, Osatomi K, Yoshida A, Osako K, Yamaguchi A, Hara K (2008) Food Chem 108:49–54
Hwang JH, Mizuta S, Yokoyama Y, Yoshinaka R (2007) Food Chem 100:921–925
Nomura Y (2004) Dev Food Sci 42:147–158
AOAC (2000) Official methods of analysis. Association of Official Analytical Chemists Inc, Arlington
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H (2008) J Food Sci 73:C413–C419
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) J Biol Chem 193:265–275
Kittiphattanabawon P, Benjakul S, Visessanguan W, Nagai T, Tanaka M (2005) Food Chem 89:363–372
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H (2007) Food Chem 104:593–601
Laemmli UK (1970) Nature 227:680–685
Nagai T, Suzuki N, Nagashima T (2008) Food Chem 111:296–301
Jongjareonrak A, Benjakul S, Visessanguan W, Nagai T, Tanaka M (2005) Food Chem 93:475–484
Duan R, Zhang J, Du X, Yao X, Konno K (2009) Food Chem 112:702–706
Foegeding EA, Lanier TC, Hultin HO (1996) In: Fennema OR (ed) Food chemistry. Marcel Dekker, New York
Muyonga JH, Cole CGB, Duodu KG (2004) Food Chem 85:81–89
Kimura S, Kamimura T, Takema Y, Kubota M (1981) BBA Protein Struct 669:251–257
Jekel PA, Weijer WJ, Beintema JJ (1983) Anal Biochem 134:347–354
Vercaigne-Marko D, Kosciarz E, Nedjar-Arroume N, Guillochon D (2000) Biotechnol Appl Biochem 31:127–134
Wang L, An X, Xin Z, Zhao L, Hu Q (2007) J Food Sci 72:E450–E455
Damodaran S (1996) In: Fennema OR (ed) Food chemistry, Marcel Dekker, New York
Muyonga JH, Cole CGB, Duodu KG (2004) Food Chem 86:325–332
Payne KJ, Veis A (1988) Biopolymers 27:1749–1760
Surewicz WK, Mantsh HH (1988) Biochim Biophys Acta 952:115–130
Plepis AMDG, Goissis G, Das-Gupta DK (1996) Polym Eng Sci 36:2932–2938
Doyle BB, Blout ER, Bendit EG (1975) Biopolymers 14:937–957
Acknowledgments
This research was supported by grant from under the program Strategic Scholarships for Frontier Research Network for the Joint PhD Program Thai Doctoral degree from the Office of the Higher Education Commission, Thailand. Authors would like to thank the Prince of Songkla University for the financial support, Professor Dr. Hideki Kishimura of laboratory of Marine Products and Food Science, Research Faculty of Fisheries Sciences, Hokkaido University, Japan, for his assistance in amino acid analysis and Mr. Veera Prateeptintong, a managing director of Blue Ocean Food Products, Co., Ltd., Samutsakhon Province, Thailand, for kindly providing blacktip shark skin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kittiphattanabawon, P., Benjakul, S., Visessanguan, W. et al. Isolation and properties of acid- and pepsin-soluble collagen from the skin of blacktip shark (Carcharhinus limbatus). Eur Food Res Technol 230, 475–483 (2010). https://doi.org/10.1007/s00217-009-1191-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-009-1191-0