Abstract
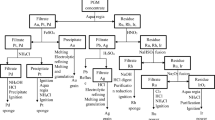
A new method for the quantitative extraction and determination of trace amounts of iridium from hydrochloric acid media has been established based on the formation of an ion-association complex of iridium hexachloro anion IrCl6 2– with dicyclohexyl-18-crown-6 (DC18C6) oxonium cation in chloroform, then determination by inductively coupled plasma atomic emission spectrometry (ICP–AES). The effect of various factors (solvent, acid concentration, crown ether, reagent concentration, shaking time, composition of the extracted species, foreign ions, etc.) on the extraction and back-extraction of iridium has been investigated. The procedure was used to determine traces of iridium in palladium chloride and rhodium chloride.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 16 October 2000 / Revised: 27 November 2000 / Accepted: 4 December 2000
Rights and permissions
About this article
Cite this article
Hossain, K., Camagong, C. & Honjo, T. Extraction of iridium(IV) from hydrochloric acid media with crown ether in chloroform, and its determination by ICP–AES. Fresenius J Anal Chem 369, 543–545 (2001). https://doi.org/10.1007/s002160000689
Issue Date:
DOI: https://doi.org/10.1007/s002160000689