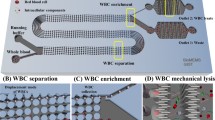
Abstract
Conventional DNA sample preparation methods involve tedious sample handling steps that require numerous inhibitors of the polymerase chain reaction (PCR) and instrumentation to implement. These disadvantages limit the applicability of conventional cell lysis and DNA extraction methods in high-throughput applications, particularly in forensics and clinical laboratories. To overcome these drawbacks, a series of nine hydrophobic magnetic ionic liquids (MILs) previously shown to preconcentrate DNA were explored as cell lysis reagents. The MILs were found to lyse white blood cells from whole blood, 2-fold diluted blood, and dry blood samples while simultaneously extracting human genomic DNA. The identity of metal ion incorporated within the MIL appears to cause hemolysis while the cationic component further reduces the cell’s integrity. Over 500 pg of human genomic DNA was isolated from 50 μL of whole blood using the trioctylbenzylammonium tris(hexafluoroacetylaceto)nickelate(II) ([N8,8,8,Bz+][Ni(hfacac)3−]) MIL, and 800 pg DNA was isolated from a dry blood samples using the trihexyl(tetradecyl)phosphonium tris(phenyltrifluoroacetylaceto)nickelate(II) ([P6,6,6,14+][Ni(Phfacac)3−]) MIL following a 1-min vortex step. A rapid, one-step cell lysis and DNA extraction from blood is ideal for settings that seek high-throughput analysis while minimizing the potential for contamination.
Graphical abstract







Similar content being viewed by others
References
Stramer SL, Wend U, Candotti D, Foster GA, Hollinger FB, Dodd RY, et al. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med. 2011;364:236–47. https://doi.org/10.1056/NEJMoa1007644.
Gonçalves J, Moreira E, Sequeira IJ, Rodrigues AS, Rueff J, Brás A. Integration of HIV in the human genome: which sites are preferential? A genetic and statistical assessment. Int J Genomics. 2016;2016:8–13. https://doi.org/10.1155/2016/2168590.
Choi J, Hyun JC, Yang S. On-chip extraction of intracellular molecules in white blood cells from whole blood. Sci Rep. 2015;5:1–12. https://doi.org/10.1038/srep15167.
Albariño CG, Romanowski V. Phenol extraction revisited: a rapid method for the isolation and preservation of human genomic DNA from whole blood. Mol Cell Probes. 1994;8:423–7.
Chen X, Cui D, Liu C, Li H, Chen J. Continuous flow microfluidic device for cell separation, cell lysis and DNA purification. Anal Chim Acta. 2007;584:237–43. https://doi.org/10.1016/j.aca.2006.11.057.
Ali N, Rampazzo RDCP, ADiT C, Krieger MA. Current nucleic acid extraction methods and their implications to point-of-care diagnostics. Biomed Res Int. 2017;2017. https://doi.org/10.1155/2017/9306564.
Nan L, Jiang Z, Wei X. Emerging microfluidic devices for cell lysis: a review. Lab Chip. 2014;14:1060–73. https://doi.org/10.1039/c3lc51133b.
Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors - occurrence, properties and removal. J Appl Microbiol. 2012;113:1014–26. https://doi.org/10.1111/j.1365-2672.2012.05384.x.
Fuchs-Telka S, Fister S, Mester PJ, Wagner M, Rossmanith P. Hydrophobic ionic liquids for quantitative bacterial cell lysis with subsequent DNA quantification. Anal Bioanal Chem. 2017;409:1503–11. https://doi.org/10.1007/s00216-016-0112-x.
Fister S, Fuchs S, Mester P, Kilpeläinen I, Wagner M, Rossmanith P. The use of ionic liquids for cracking viruses for isolation of nucleic acids. Sep Purif Technol. 2015;155:38–44. https://doi.org/10.1016/j.seppur.2015.03.035.
Martzy R, Bica-Schröder K, Pálvölgyi ÁM, Kolm C, Jakwerth S, Kirschner AKT, et al. Simple lysis of bacterial cells for DNA-based diagnostics using hydrophilic ionic liquids. Sci Rep. 2019;9:1–10. https://doi.org/10.1038/s41598-019-50246-5.
Trujillo-Rodriguez MJ, Nan H, Varona M, Emaus MN, Souza ID, Anderson JL. Advances of ionic liquids in analytical chemistry. Anal Chem. 2019;91:505–31. https://doi.org/10.1021/acs.analchem.8b04710.
Trujillo-Rodríguez MJ, Rocío-Bautista P, Pino V, Afonso AM. Ionic liquids in dispersive liquid-liquid microextraction. TrAC - Trends Anal Chem. 2013;51:87–106. https://doi.org/10.1016/j.trac.2013.06.008.
Koshy L, Anju AL, Harikrishnan S, Kutty VR, Jissa VT, Kurikesu I, et al. Evaluating genomic DNA extraction methods from human whole blood using endpoint and real-time PCR assays. Mol Biol Rep. 2017;44:97–108. https://doi.org/10.1007/s11033-016-4085-9.
Nanayakkara IA, Cao W, White IM. Simplifying nucleic acid amplification from whole blood with direct polymerase chain reaction on chitosan microparticles. Anal Chem. 2017;89:3773–9. https://doi.org/10.1021/acs.analchem.7b00274.
Berasaluce A, Matthys L, Mujika J, Antoñana-Díez M, Valero A, Agirregabiria M. Bead beating-based continuous flow cell lysis in a microfluidic device. RSC Adv. 2015;5:22350–5. https://doi.org/10.1039/c5ra01251a.
Pandit KR, Nanayakkara IA, Cao W, Raghavan SR, White IM. Capture and direct amplification of DNA on chitosan microparticles in a single PCR-optimal solution. Anal Chem. 2015;87:11022–9. https://doi.org/10.1021/acs.analchem.5b03006.
Clark KD, Nacham O, Yu H, Li T, Yamsek MM, Ronning DR, et al. Extraction of DNA by magnetic ionic liquids: tunable solvents for rapid and selective DNA analysis. Anal Chem. 2015;87:1552–9. https://doi.org/10.1021/ac504260t.
Marengo A, Cagliero C, Sgorbini B, Anderson JL, Emaus MN, Bicchi C, et al. Development of an innovative and sustainable one-step method for rapid plant DNA isolation for targeted PCR using magnetic ionic liquids. Plant Methods. 2019:1–11. https://doi.org/10.1186/s13007-019-0408-x.
Bowers AN, Trujillo-Rodríguez MJ, Farooq MQ, Anderson JL. Extraction of DNA with magnetic ionic liquids using in situ dispersive liquid–liquid microextraction. Anal Bioanal Chem. 2019;411:7375–85. https://doi.org/10.1007/s00216-019-02163-9.
Clark KD, Nacham O, Purslow JA, Pierson SA, Anderson JL. Magnetic ionic liquids in analytical chemistry: a review. Anal Chim Acta. 2016;934:9–21. https://doi.org/10.1016/j.aca.2016.06.011.
Del Sesto RE, McCleskey TM, Burrell AK, Baker GA, Thompson JD, Scott BL, et al. Structure and magnetic behavior of transition metal based ionic liquids. Chem Commun. 2008:447–9. https://doi.org/10.1039/b711189d.
Hayashi S, Hamaguchi H. Discovery of a magnetic ionic liquid [bmim]FeCl4. Chem Lett. 2004;33:1590–1. https://doi.org/10.1246/cl.2004.1590.
Clark KD, Yamsek MM, Nacham O, Anderson JL. Magnetic ionic liquids as PCR-compatible solvents for DNA extraction from biological samples. Chem Commun. 2015;51:16771–3. https://doi.org/10.1039/C5CC07253K.
Emaus MN, Anderson JL. Allelic discrimination between circulating tumor DNA fragments enabled by a multiplex-qPCR assay containing DNA-enriched magnetic ionic liquids. Anal Chim Acta. 2020;1124:184–93. https://doi.org/10.1016/j.aca.2020.04.078.
Emaus MN, Clark KD, Hinners P, Anderson JL. Preconcentration of DNA using magnetic ionic liquids that are compatible with real-time PCR for rapid nucleic acid quantification. Anal Bioanal Chem. 2018;410:4135–44.
Pierson SA, Nacham O, Clark KD, Nan H, Mudryk Y, Anderson JL. Synthesis and characterization of low viscosity hexafluoroacetylacetonate-based hydrophobic magnetic ionic liquids. New J Chem. 2017;41:5498–505. https://doi.org/10.1039/c7nj00206h.
Farooq MQ, Chand D, Odugbesi GA, Varona M, Mudryk Y, Anderson JL. Investigating the effect of ligand and cation on the properties of metal fluorinated acetylacetonate based magnetic ionic liquids. New J Chem. 2019;43:11334–41. https://doi.org/10.1039/C9NJ02595B.
Strober W. Wright-Giemsa and nonspecific esterase staining of cells. Curr Protoc Cytom. 2000;11. https://doi.org/10.1002/0471142956.cya03ds11.
Whitehouse CA, Hottel HE. Comparison of five commercial DNA extraction kits for the recovery of Francisella tularensis DNA from spiked soil samples. Mol Cell Probes. 2007;21:92–6. https://doi.org/10.1016/j.mcp.2006.08.003.
Clark KD, Sorensen M, Nacham O, Anderson JL. Preservation of DNA in nuclease-rich samples using magnetic ionic liquids. RSC Adv. 2016;6:39846–51. https://doi.org/10.1039/C6RA05932E.
Silber R. Of acanthocytes, spurs, burrs, and membranes. Blood. 1969;34:111–4.
Owen JS, Brown DJC, Harry DS, McIntyre N, Beaven GH, Isenberg H, et al. Erythrocyte echinocytosis in liver disease. Role of abnormal plasma high density lipoproteins. J Clin Invest. 1985;76:2275–85. https://doi.org/10.1172/JCI112237.
Salvioli G, Rioli G, Lugli R, Salati R. Membrane lipid composition of red blood cells in liver disease: regression of spur cell anaemia after infusion of polyunsaturated phosphatidylcholine. Gut. 1978;19:844–50. https://doi.org/10.1136/gut.19.9.844.
Beck JS. Echinocyte formation: a test case for mechanisms of cell shape changes. J Theor Biol. 1978;71:515–24. https://doi.org/10.1016/0022-5193(78)90322-3.
Ribarov SR, Benov LC. Relationship between the hemolytic action of heavy metals and lipid peroxidation. BBA - Biomembr. 1981;640:721–6. https://doi.org/10.1016/0005-2736(81)90102-4.
Shalel S, Streichman S, Marmur A. The mechanism of hemolysis by surfactants: effect of solution composition. J Colloid Interface Sci. 2002;252:66–76. https://doi.org/10.1006/jcis.2002.8474.
Manaargadoo-Catin M, Ali-Cherif A, Pougnas JL, Perrin C. Hemolysis by surfactants - a review. Adv Colloid Interf Sci. 2016;228:1–16. https://doi.org/10.1016/j.cis.2015.10.011.
Acknowledgments
M. N. E. would like to thank Paul M. Emaus for his support. J. L. A. acknowledges funding from the Chemical Measurement and Imaging Program at the National Science Foundation (CHE-1709372).
Funding
This study was funded by the Chemical Measurement and Imaging Program at the National Science Foundation (CHE-1709372).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article contains studies involving human blood samples that were purchased commercially. All studies and procedures have been approved by the Institutional Biosafety Committee (IBC) at Iowa State University.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 746 kb)
Rights and permissions
About this article
Cite this article
Emaus, M.N., Anderson, J.L. Simultaneous cell lysis and DNA extraction from whole blood using magnetic ionic liquids. Anal Bioanal Chem 412, 8039–8049 (2020). https://doi.org/10.1007/s00216-020-02941-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02941-w