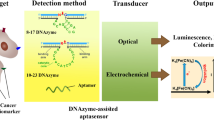
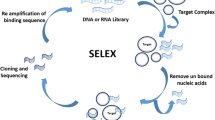
Abstract
Aptamers are chemically synthetic single-stranded DNA or RNA molecules selected by molecular evolution. They have been widely used as attractive tools in biosensing and bioimaging because they can bind to a large variety of targets with high sensitivity and high affinity and specificity. As recognition elements, aptamers contribute in particular to cancer diagnostics by recognizing different cancer biomarkers, while they can also facilitate ultrasensitive detection by further employing signal amplification elements. Optical techniques have been widely used for direct and real-time monitoring of cancer-related biomolecules and bioprocesses due to the high sensitivity, quick response, and simple operation, which has greatly benefited cancer diagnostics. In this review, we highlight recent advances in optical platform-based sensing strategies for cancer diagnostics aided by aptamers. Limitations and current challenges are also discussed.








Similar content being viewed by others
References
Zuo XL, Song SP, Zhang J, Pan D, Wang LH, Fan CH. A target-responsive electrochemical aptamer switch (TREAS) for reagentless detection of nanomolar ATP. J Am Chem Soc. 2007;129(5):1042–3.
Liu Q, Peng YJ, Xu JC, Ma C, Li LL, Mao CJ, et al. Label-free Electrochemiluminescence Aptasensor for highly sensitive detection of Acetylcholinesterase based on au-nanoparticle-functionalized g-C3N4 Nanohybrid. Chemelectrochem. 2017;4(7):1768–74.
Cao Y, Chen DH, Chen WW, Yu JC, Chen Z, Li GX. Aptamer-based homogeneous protein detection using cucurbit[7]uril functionalized electrode. Anal Chim Acta. 2014;812:45–9.
Miao P, Tang YG, Wang BD, Han K, Chen XF, Sun HX. An aptasensor for detection of potassium ions based on RecJ(f) exonuclease mediated signal amplification. Analyst. 2014;139(22):5695–9.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment - Rna ligands to bacteriophage-T4 DNA-polymerase. Science. 1990;249(4968):505–10.
Ellington AD, Szostak JW. Invitro selection of Rna molecules that bind specific ligands. Nature. 1990;346(6287):818–22.
Meng FY, Han K, Wang BD, Liu T, Liu GX, Li YR, et al. Nanoarchitectured electrochemical Cytosensor for selective detection of Cancer cells. Chemistryselect. 2016;1(7):1515–7.
Sheng LL, Lu YH, Deng S, Liao XY, Zhang KX, Ding T, et al. A transcription aptasensor: amplified, label-free and culture-independent detection of foodborne pathogens via light-up RNA aptamers. Chem Commun. 2019;55(68):10096–9.
Wang J, Cheng WB, Meng FY, Yang M, Pan Y, Miao P. Hand-in-hand RNA nanowire-based aptasensor for the detection of theophylline. Biosens Bioelectron. 2018;101:153–8.
Morozova N, Allers J, Myers J, Shamoo Y. Protein-RNA interactions: exploring binding patterns with a three-dimensional superposition analysis of high resolution structures. Bioinformatics. 2006;22(22):2746–52.
Zimmermann GR, Jenison RD, Wick CL, Simorre JP, Pardi A. Interlocking structural motifs mediate molecular discrimination by a theophylline-binding RNA. Nat Struct Biol. 1997;4(8):644–9.
Wen YL, Pei H, Wan Y, Su Y, Huang Q, Song SP, et al. DNA nanostructure-decorated surfaces for enhanced Aptamer-target binding and electrochemical cocaine sensors. Anal Chem. 2011;83(19):7418–23.
Liu HY, Xu SM, He ZM, Deng AP, Zhu JJ. Supersandwich Cytosensor for selective and ultrasensitive detection of Cancer cells using Aptamer-DNA Concatamer-quantum dots probes. Anal Chem. 2013;85(6):3385–92.
Gu HD, Yang YY, Chen F, Liu TT, Jin J, Pan Y, et al. Electrochemical detection of arsenic contamination based on hybridization chain reaction and RecJ(f) exonuclease-mediated amplification. Chem Eng J. 2018;353:305–10.
Huang Y, Li H, Wang L, Mao XX, Li GX. Highly sensitive protein detection based on smart hybrid Nanocomposite-controlled switch of DNA polymerase activity. Acs Appl Mater Inter. 2016;8(41):28202–7.
Miao P, Yang DW, Chen XF, Guo ZZ, Tang YG. Voltammetric determination of tumor necrosis factor-alpha based on the use of an aptamer and magnetic nanoparticles loaded with gold nanoparticles. Microchim Acta. 2017;184(10):3901–7.
Yang DW, Tang YG, Guo ZZ, Chen XF, Miao P. Proximity aptasensor for protein detection based on an enzyme-free amplification strategy. Mol BioSyst. 2017;13(10):1936–9.
Zhang J, Chen H, Cao Y, Feng C, Zhu XL, Li GX. Design Nanoprobe based on its binding with amino acid residues on cell surface and its application to electrochemical analysis of cells. Anal Chem. 2019;91(1):1005–10.
Cheng WB, Xu J, Guo ZZ, Yang DW, Chen XF, Yan W, et al. Hydrothermal synthesis of N,S co-doped carbon nanodots for highly selective detection of living cancer cells. J Mater Chem B. 2018;6(36):5775–80.
Miao P, Tang Y. Gold nanoparticles-based multipedal DNA Walker for Ratiometric detection of circulating tumor cell. Anal Chem. 2019;91(23):15187–92.
Cruz-Toledo J, McKeague M, Zhang XR, Giamberardino A, McConnell E, Francis T, DeRosa MC, Dumontier M (2012) Aptamer base: a collaborative knowledge base to describe aptamers and SELEX experiments. Database-Oxford.
Darmostuk M, Rimpelova S, Gbelcova H, Ruml T. Current approaches in SELEX: an update to aptamer selection technology. Biotechnol Adv. 2015;33(6):1141–61.
Stoltenburg R, Reinemann C, Strehlitz B. SELEX--a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng. 2007;24(4):381–403.
Hassan EM, DeRosa MC (2020) Recent advances in cancer early detection and diagnosis: Role of nucleic acid based aptasensors Trac-Trend Anal Chem 124.
Ma XY, Guo ZZ, Mao ZQ, Tang YG, Miao P. Colorimetric theophylline aggregation assay using an RNA aptamer and non-crosslinking gold nanoparticles. Microchim Acta. 2018;185(1).
Song S, Wang L, Li J, Fan C, Zhao J. Aptamer-based biosensors. TrAC Trends Anal Chem. 2008;27(2):108–17.
Chen XF, Guo ZZ, Tang YG, Shen Y, Miao P. A highly sensitive gold nanoparticle-based electrochemical aptasensor for theophylline detection. Anal Chim Acta. 2018;999:54–9.
Diaz-Amaya S, Lin LK, Deering AJ, Stanciu LA. Aptamer-based SERS biosensor for whole cell analytical detection of E. coli O157:H7. Anal Chim Acta. 2019;1081:146–56.
Han K, Liu T, Wang YH, Miao P. Electrochemical aptasensors for detection of small molecules, macromolecules, and cells. Rev Anal Chem. 2016;35(4):201–11.
Li F, Zhang HQ, Wang ZX, Newbigging AM, Reid MS, Li XF, et al. Aptamers facilitating amplified detection of biomolecules. Anal Chem. 2015;87(1):274–92.
Kou XY, Liu T, Yang XB, Tang YG, Gong X, Miao P. Role of Tripodal DNA modified Gold nanoparticles in colorimetric Aptasensing. Colloid Interfac Sci. 2017;21:19–21.
Miao P, Tang YG, Wang BD, Yin J, Ning LM. Signal amplification by enzymatic tools for nucleic acids. Trac-Trend Anal Chem. 2015;67:1–15.
Ferreira CSM, Papamichael K, Guilbault G, Schwarzacher T, Gariepy J, Missailidis S. DNA aptamers against the MUC1 tumour marker: design of aptamer-antibody sandwich ELISA for the early diagnosis of epithelial tumours. Anal Bioanal Chem. 2008;390(4):1039–50.
Park JH, Cho YS, Kang S, Lee EJ, Lee GH, Hah SS. A colorimetric sandwich-type assay for sensitive thrombin detection based on enzyme-linked aptamer assay. Anal Biochem. 2014;462:10–2.
Zou MJ, Wang SY. An Aptamer-based self-catalytic colorimetric assay for Carcinoembryonic antigen. B Korean Chem Soc. 2017;38(10):1143–8.
Zhu X, Cao Y, Liang Z, Li G. Aptamer-based and DNAzyme-linked colorimetric detection of cancer cells. Protein & cell. 2010;1(9):842–6.
Shahbazi N, Hosseinkhani S, Ranjbar B. A facile and rapid aptasensor based on split peroxidase DNAzyme for visual detection of carcinoembryonic antigen in saliva. Sensor Actuat B-Chem. 2017;253:794–803.
Nasir M, Nawaz MH, Latif U, Yaqub M, Hayat A, Rahim A. An overview on enzyme-mimicking nanomaterials for use in electrochemical and optical assays. Microchim Acta. 2016;184(2):1–20.
Gao LZ, Zhuang J, Nie L, Zhang JB, Zhang Y, Gu N, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577–83.
Zhang Z, Wang Z, Wang X, Yang X. Magnetic nanoparticle-linked colorimetric aptasensor for the detection of thrombin. Sensors & Actuators B Chemical. 2010;147(2):428–33.
Wang K, Fan D, Liu Y, Wang E. Highly sensitive and specific colorimetric detection of cancer cells via dual-aptamer target binding strategy. Biosens Bioelectron. 2015;73:1–6.
Xiang SY, Yang P, Guo H, Zhang S, Zhang XK, Zhu F, et al. Green tea makes polyphenol nanoparticles with radical-scavenging activities. Macromol Rapid Comm. 2017;38(23).
Wang Z, Carniato F, Xie YJ, Huang YR, Li YW, He S, et al. High Relaxivity gadolinium-polydopamine nanoparticles. Small. 2017;13(43).
Li C, Lu JY, Hu XL, Feng C, Xiang Y, Karamanos Y, et al. Assembly of nanoconjugates as new kind inhibitor of the aggregation of amyloid peptides associated with Alzheimer’s disease. Part Part Syst Charact. 2018;35(3).
Miao P, Tang YG, Mao ZQ, Liu YZ. Adamantane derivatives functionalized Gold nanoparticles for colorimetric detection of MiRNA. Part Part Syst Charact. 2017;34(6).
Huang CC, Huang YF, Cao Z, Tan W, Chang HT. Aptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptors. Anal Chem. 2005;77(17):5735–41.
Lin TE, Chen WH, Shiang YC, Huang CC, Chang HT. Colorimetric detection of platelet-derived growth factors through competitive interactions between proteins and functional gold nanoparticles. Biosens Bioelectron. 2011;29(1):204–9.
Cho EJ, Lee JW, Ellington AD. Applications of aptamers as sensors. Annu Rev Anal Chem. 2015;2(1):241–64.
Jiang YX, Fang XH, Bai CL. Signaling aptamer/protein binding by a molecular light switch complex. Anal Chem. 2004;76(17):5230–5.
Lin X, Leung KH, Lin L, Lin L, Lin S, Leung CH, et al. Determination of cell metabolite VEGF(1)(6)(5) and dynamic analysis of protein-DNA interactions by combination of microfluidic technique and luminescent switch-on probe. Biosens Bioelectron. 2016;79:41–7.
Wang K, He MQ, Zhai FH, He RH, Yu YL. A label-free and enzyme-free ratiometric fluorescence biosensor for sensitive detection of carcinoembryonic antigen based on target-aptamer complex recycling amplification. Sensor Actuat B-Chem. 2017;253:893–9.
Zhao L, Tang C, Xu L, Zhang Z, Li X, Hu H, et al. Enhanced and differential capture of circulating tumor cells from lung Cancer patients by microfluidic assays using Aptamer cocktail. Small. 2016;12(8):1072–81.
Miao P, Han K, Tang YG, Wang BD, Lin T, Cheng WB. Recent advances in carbon nanodots: synthesis, properties and biomedical applications. Nanoscale. 2015;7(5):1586–95.
Jiang YT, Ma XY, Shao XJ, Wang MY, Jiang Y, Miao P. Chameleon silver nanoclusters for ratiometric sensing of miRNA. Sensor Actuat B-Chem. 2019;297:126788.
Yang YY, Wang XF, Liao GC, Liu XQ, Chen QL, Li HM, et al. iRGD-decorated red shift emissive carbon nanodots for tumor targeting fluorescence imaging. J Colloid Interf Sci. 2018;509:515–21.
Sun SJ, Guan QW, Liu Y, Wei B, Yang YY, Yu ZQ. Highly luminescence manganese doped carbon dots. Chinese Chem Lett. 2019;30(5):1051–4.
Motaghi H, Mehrgardi MA, Bouvet P. Carbon dots-AS1411 Aptamer Nanoconjugate for ultrasensitive Spectrofluorometric detection of Cancer cells. Sci Rep. 2017;7(1):10513.
Yin J, He X, Wang K, Xu F, Shangguan J, He D, et al. Label-free and turn-on aptamer strategy for cancer cells detection based on a DNA-silver nanocluster fluorescence upon recognition-induced hybridization. Anal Chem. 2013;85(24):12011–9.
Jiang YT, Tang YG, Miao P. Polydopamine nanosphere@silver nanoclusters for fluorescence detection of multiplex tumor markers. Nanoscale. 2019;11(17):8119–23.
Jo H, Her J, Ban C. Dual aptamer-functionalized silica nanoparticles for the highly sensitive detection of breast cancer. Biosens Bioelectron. 2015;71:129–36.
Smith JE, Medley CD, Tang Z, Shangguan D, Lofton C, Tan W. Aptamer-conjugated nanoparticles for the collection and detection of multiple cancer cells. Anal Chem. 2007;79(8):3075–82.
Hu ZX, Tan JT, Lai ZQ, Zheng R, Zhong JH, Wang YW, Li XX, Yang N, Li JP, Yang W, Huang Y, Zhao YX, Lu XL (2017) Aptamer Combined with Fluorescent Silica Nanoparticles for Detection of Hepatoma Cells Nanoscale Res Lett 12.
Sheng ZH, Hu DH, Zhang PF, Gong P, Gao DY, Liu SH, et al. Cation exchange in aptamer-conjugated CdSe nanoclusters: a novel fluorescence signal amplification for cancer cell detection. Chem Commun. 2012;48(35):4202–4.
Meng FY, Chai H, Ma XY, Tang YG, Miao P. FRET investigation toward DNA tetrahedron-based ratiometric analysis of intracellular telomerase activity. J Mater Chem B. 2019;7(11):1926–32.
Hamdghadareh S, Salimi A, Fathi F, Bahrami S. An amplified comparative fluorescence resonance energy transfer immunosensing of CA125 tumor marker and ovarian cancer cells using green and economic carbon dots for bio-applications in labeling, imaging and sensing. Biosens Bioelectron. 2017;96:308–16.
Pereira M, Tome D, Domingues AS, Varanda AS, Paulo C, Santos MAS, et al. A fluorescence-based sensor assay that monitors general protein aggregation in human cells. Biotechnol J. 2018;13(4):–e1700676.
Kopra K, Syrjanpaa M, Hanninen P, Harma H. Non-competitive aptamer-based quenching resonance energy transfer assay for homogeneous growth factor quantification. Analyst. 2014;139(8):2016–23.
Wang Y, Bao L, Liu Z, Pang DW. Aptamer biosensor based on fluorescence resonance energy transfer from upconverting phosphors to carbon nanoparticles for thrombin detection in human plasma. Anal Chem. 2011;83(21):8130–7.
Huang CC, Chiu SH, Huang YF, Chang HT. Aptamer-functionalized gold nanoparticles for turn-on light switch detection of platelet-derived growth factor. Anal Chem. 2007;79(13):4798–804.
Yang T, Hou P, Zheng LL, Zhan L, Gao PF, Li YF, et al. Surface-engineered quantum dots/electrospun nanofibers as a networked fluorescence aptasensing platform toward biomarkers. Nanoscale. 2017;9(43):17020–8.
Shi H, He XX, Wang KM, Wu X, Ye XS, Guo QP, et al. Activatable aptamer probe for contrast-enhanced in vivo cancer imaging based on cell membrane protein-triggered conformation alteration. P Natl Acad Sci USA. 2011;108(10):3900–5.
Zhao B, Wu P, Zhang H, Cai C. Designing activatable aptamer probes for simultaneous detection of multiple tumor-related proteins in living cancer cells. Biosens Bioelectron. 2015;68:763–70.
Kong RM, Ding L, Wang Z, You J, Qu F. A novel aptamer-functionalized MoS2 nanosheet fluorescent biosensor for sensitive detection of prostate specific antigen. Anal Bioanal Chem. 2015;407(2):369–77.
Niazi JH, Verma SK, Niazi S, Qureshi A. In vitro HER2 protein-induced affinity dissociation of carbon nanotube-wrapped anti-HER2 aptamers for HER2 protein detection. Analyst. 2015;140(1):243–9.
Li H, Shi L, Sun DE, Li PW, Liu ZH. Fluorescence resonance energy transfer biosensor between upconverting nanoparticles and palladium nanoparticles for ultrasensitive CEA detection. Biosens Bioelectron. 2016;86:791–8.
Wang H, Chen H, Huang Z, Li T, Deng A, Kong J. DNase I enzyme-aided fluorescence signal amplification based on graphene oxide-DNA aptamer interactions for colorectal cancer exosome detection. Talanta. 2018;184:219–26.
Yan L, Shi H, He X, Wang K, Tang J, Chen M, et al. A versatile activatable fluorescence probing platform for cancer cells in vitro and in vivo based on self-assembled aptamer/carbon nanotube ensembles. Anal Chem. 2014;86(18):9271–7.
Fang BY, Wang CY, Li C, Wang HB, Zhao YD. Amplified using DNase I and aptamer/graphene oxide for sensing prostate specific antigen in human serum. Sensor Actuat B-Chem. 2017;244:928–33.
Hao TT, Wu XL, Xu LG, Liu LQ, Ma W, Kuang H, et al. Ultrasensitive detection of prostate-specific antigen and thrombin based on Gold-Upconversion nanoparticle assembled pyramids. Small. 2017;13(19).
Das P, Krull UJ (2017) Detection of a cancer biomarker protein on modified cellulose paper by fluorescence using aptamer-linked quantum dots. Analyst 142.
Deng YL, Xu DD, Pang DW, Tang HW. Target-triggered signal turn-on detection of prostate specific antigen based on metal-enhanced fluorescence of Ag@SiO2@SiO2-RuBpy composite nanoparticles. Nanotechnology. 2017;28(6):065501.
Kim GI, Kim KW, Oh MK, Sung YM. The detection of platelet derived growth factor using decoupling of quencher-oligonucleotide from aptamer/quantum dot bioconjugates. Nanotechnology. 2009;20(17):175503.
Li H, Sun DE, Liu YJ, Liu ZH. An ultrasensitive homogeneous aptasensor for kanamycin based on upconversion fluorescence resonance energy transfer. Biosens Bioelectron. 2014;55:149–56.
Park L, Kim J, Lee JH. Role of background observed in aptasensor with chemiluminescence detection. Talanta. 2013;116:736–42.
Pasquardini L, Pancheri L, Potrich C, Ferri A, Piemonte C, Lunelli L, et al. SPAD aptasensor for the detection of circulating protein biomarkers. Biosens Bioelectron. 2015;68:500–7.
Bi S, Luo BY, Ye JY, Wang ZH. Label-free chemiluminescent aptasensor for platelet-derived growth factor detection based on exonuclease-assisted cascade autocatalytic recycling amplification. Biosens Bioelectron. 2014;62:208–13.
Wang P, Song YH, Zhao YJ, Fan AP. Hydroxylamine amplified gold nanoparticle-based aptameric system for the highly selective and sensitive detection of platelet-derived growth factor. Talanta. 2013;103:392–7.
Yao LY, Yu XQ, Zhao YJ, Fan AP. An aptamer-based chemiluminescence method for ultrasensitive detection of platelet-derived growth factor by cascade amplification combining rolling circle amplification with hydroxylamine-enlarged gold nanoparticles. Anal Methods-Uk. 2015;7(20):8786–92.
Zhang XF, Zhang H, Xu SX, Sun YH. A highly sensitive LED-induced chemiluminescence platform for aptasensing of platelet-derived growth factor. Analyst. 2014;139(1):133–7.
Lee JH, Rho JER, Rho THD, Newby JG. Advent of innovative chemiluminescent enzyme immunoassay. Biosens Bioelectron. 2010;26(2):377–82.
Choi HK, Lee JH. Role of magnetic Fe3O4 graphene oxide in chemiluminescent aptasensors capable of sensing tumor markers in human serum. Anal Methods-Uk. 2013;5(24):6964–8.
Khang H, Cho K, Chong S, Lee JH. All-in-one dual-aptasensor capable of rapidly quantifying carcinoembryonic antigen. Biosens Bioelectron. 2017;90:46–52.
Shi GF, Cao JT, Zhang JJ, Huang KJ, Liu YM, Chen YH, et al. Aptasensor based on tripetalous cadmium sulfide-graphene electrochemiluminescence for the detection of carcinoembryonic antigen. Analyst. 2014;139(22):5827–34.
Zhang H, Li M, Li C, Guo Z, Dong H, Wu P, et al. G-quadruplex DNAzyme-based electrochemiluminescence biosensing strategy for VEGF165 detection: combination of aptamer-target recognition and T7 exonuclease-assisted cycling signal amplification. Biosens Bioelectron. 2015;74:98–103.
Cao JT, Yang JJ, Zhao LZ, Wang YL, Wang H, Liu YM, et al. Graphene oxide@gold nanorods-based multiple-assisted electrochemiluminescence signal amplification strategy for sensitive detection of prostate specific antigen. Biosens Bioelectron. 2018;99:92–8.
Wu MS, Liu Z, Shi HW, Chen HY, Xu JJ. Visual Electrochemiluminescence detection of Cancer biomarkers on a closed bipolar electrode Array Chip. Anal Chem. 2015;87(1):530–7.
Zhu D, Zhou X, Xing D. A new kind of aptamer-based immunomagnetic electrochemiluminescence assay for quantitative detection of protein. Biosens Bioelectron. 2010;26(1):285–8.
Chen M, Bi S, Jia X, He P. Aptamer-conjugated bio-bar-code au-Fe3O4 nanoparticles as amplification station for electrochemiluminescence detection of tumor cells. Anal Chim Acta. 2014;837:44–51.
Ma W, Yin H, Xu L, Wu X, Kuang H, Wang L, et al. Ultrasensitive aptamer-based SERS detection of PSAs by heterogeneous satellite nanoassemblies. Chem Commun (Camb). 2014;50(68):9737–40.
Yang K, Hu Y, Dong N, Zhu G, Zhu T, Jiang N. A novel SERS-based magnetic aptasensor for prostate specific antigen assay with high sensitivity. Biosens Bioelectron. 2017;94:286–91.
Xu C, Wu X, Fu P, Ma W, Xu L, Kuang H. SERS-active silver nanoparticle Trimers for sub-Attomolar detection of alpha fetoprotein. RSC Adv. 2015;5(90):73395–8.
Zheng J, Hu Y, Bai J, Ma C, Li J, Li Y, et al. Universal surface-enhanced Raman scattering amplification detector for ultrasensitive detection of multiple target analytes. Anal Chem. 2014;86(4):2205–12.
Yarbakht M, Nikkhah M, Moshaii A, Weber K, Matthäus C, Cialla-May D, et al. Simultaneous isolation and detection of single breast cancer cells using surface- enhanced Raman spectroscopy. Talanta. 2018;186:44–52.
Chen ZG, Lei YL, Chen X, Wang Z, Liu JB. An aptamer based resonance light scattering assay of prostate specific antigen. Biosens Bioelectron. 2012;36(1):35–40.
Luo YH, Zhang XH, Yao DM, Wen GQ, Liu QY, Liang AH, et al. Resonance Rayleigh scattering detection of trace PDGF based on catalysis of an aptamer-modified nanogold probe in the Fehling reaction. RSC Adv. 2014;4(53):28052–5.
Gong P, Shi B, Zheng M, Wang B, Zhang P, Hu D, et al. PEI protected aptamer molecular probes for contrast-enhanced in vivo cancer imaging. Biomaterials. 2012;33(31):7810–7.
Pu Y, Liu ZX, Lu Y, Yuan P, Liu J, Yu B, et al. Using DNA Aptamer probe for Immunostaining of Cancer frozen tissues. Anal Chem. 2015;87(3):1919–24.
Li J, Dai Y, Wang S, Han C, Xu K. Aptamer-tagged green- and yellow-emitting fluorescent silver nanoclusters for specific tumor cell imaging. Sensors & Actuators B Chemical. 2016;232:1–8.
Ai J, Li T, Li BL, Xu YH, Li D, Liu ZJ, et al. In situ labeling and imaging of cellular protein via a bi-functional anticancer aptamer and its fluorescent ligand. Anal Chim Acta. 2012;741:93–9.
Xiao H, Chen YQ, Yuan EF, Li W, Jiang ZR, Wei L, et al. Obtaining more accurate signals: spatiotemporal imaging of Cancer sites enabled by a Photoactivatable Aptamer-based strategy. Acs Appl Mater Inter. 2016;8(36):23542–8.
Bagalkot V, Zhang L, Levynissenbaum E, Jon S, Robert Langer A. Quantum dot−Aptamer conjugates for synchronous Cancer imaging, therapy, and sensing of drug delivery based on Bi-fluorescence resonance energy transfer. Nano Lett. 2007;7(10):3065–70.
Zeng ZH, Parekh P, Li Z, Shi ZZ, Tung CH, Zu YL. Specific and sensitive tumor imaging using biostable oligonucleotide Aptamer probes. Theranostics. 2014;4(9):945–52.
Chen HY, Zhao J, Zhang M, Yang HB, Ma YX, Gu YQ. MUC1 Aptamer-based near-infrared fluorescence probes for tumor imaging. Mol Imaging Biol. 2015;17(1):38–48.
Pascual L, Cerqueira-Coutinho C, Garcia-Fernandez A, de Luis B, Bernardes ES, Albernaz MS, et al. MUC1 aptamer-capped mesoporous silica nanoparticles for controlled drug delivery and radio-imaging applications. Nanomed-Nanotechnol. 2017;13(8):2495–505.
Da Pieve C, Perkins AC, Missailidis S. Anti-MUC1 aptamers: radiolabelling with Tc-99m and biodistribution in MCF-7 tumour-bearing mice. Nucl Med Biol. 2009;36(6):703–10.
Borbas KE, Ferreira CSM, Perkins A, Bruce JI, Missailidis S. Design and synthesis of mono- and multimeric targeted radiopharmaceuticals based on novel cyclen ligands coupled to anti-MUC1 aptamers for the diagnostic imaging and targeted radiotherapy of cancer. Bioconjug Chem. 2007;18(4):1205–12.
Da Pieve C, Iley JN, Perkins A, Missailidis S. Development of anti-MUC1 DNA aptamers for the imaging and radiotherapy of breast cancer. Breast Cancer Res. 2008;10:S31–2.
Azhdarzadeh M, Atyabi F, Saei AA, Varnamkhasti BS, Omidi Y, Fateh M, et al. Theranostic MUC-1 aptamer targeted gold coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging and photothermal therapy of colon cancer. Colloid Surface B. 2016;143:224–32.
Yu MK, Kim D, Lee IH, So JS, Jeong YY, Jon S. Image-guided prostate Cancer therapy using Aptamer-functionalized thermally cross-linked Superparamagnetic Iron oxide nanoparticles. Small. 2011;7(15):2241–9.
Kang WJ, Lee J, Lee YS, Cho S, Ali BA, Al-Khedhairy AA, et al. Multimodal imaging probe for targeting cancer cells using uMUC-1 aptamer. Colloid Surface B. 2015;136:134–40.
Funding
This work is supported by the National Natural Science Foundation of China (grant nos. 8150140715, 81503463, 81801792), the Natural Science Foundation of Jiangsu Province of China (grant no. BK20170389), and the Science and Technology Program of Suzhou (SS201867).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kou, X., Zhang, X., Shao, X. et al. Recent advances in optical aptasensor technology for amplification strategies in cancer diagnostics. Anal Bioanal Chem 412, 6691–6705 (2020). https://doi.org/10.1007/s00216-020-02774-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02774-7