Abstract
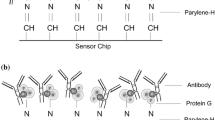
In this study, we report a direct surface plasmon resonance (SPR) biosensor based on an oriented assembly of antibody for the rapid detection of chlorpyrifos residue in agricultural samples. In this covalent-orientated strategy, staphylococcal protein A (SPA) was first covalently bound to the surface for monitoring chlorpyrifos residue, with subsequent binding of the antibody in an orientated fashion via its fragment crystallizable (Fc) region. Consequently, the SPA-modified biosensor exhibited a satisfactory specificity and a low detection limit of 0.056 ng mL−1 for chlorpyrifos, with a linear detection range of 0.25–50.0 ng mL−1. Under optimal conditions, the sensor chip could be regenerated for at least 210 cycles. The results presented here indicate that the SPA-modified sensor chip can successfully improve the sensitivity and obviating the need of the modification of the antibody. The developed SPR biosensor method has the great potential for rapid, sensitive, and specific detection with broad applications in areas of environmental monitoring and food safety.

Graphical abstract





Similar content being viewed by others
References
Harshit D, Charmy K, Nrupesh P. Organophosphorus pesticides determination by novel HPLC and spectrophotometric method. Food Chem. 2017;230:448–53.
Gupta SC, Mishra M, Sharma A, Deepak Balaji TG, Kumar R, Mishra RK, et al. Chlorpyrifos induces apoptosis and DNA damage in Drosophila through generation of reactive oxygen species. Ecotoxicol Environ Saf. 2010;73(6):1415–23.
Sotomayor V, Lascano C, D'Angelo AMP, Venturino A. Developmental and polyamine metabolism alterations in Rhinella arenarum embryos exposed to the organophosphate chlorpyrifos. Environ Toxicol Chem. 2012;31:2052–8.
Khalil AM. Toxicological effects and oxidative stress responses in freshwater snail, Lanistes carinatus, following exposure to chlorpyrifos. Ecotoxicol Environ Saf. 2015;116:137–42.
Lee W, Byung Keun O, Min Bae Y, Paek SH, Hong Lee W, Choi JW. Fabrication of self-assembled protein a monolayer and its application as an immunosensor. Biosens Bioelectron. 2003;19(3):185–92.
Dou XW, Zhang L, Liu C, Li Q, Luo J, Yang MH. Fluorometric competitive immunoassay for chlorpyrifos using rhodamine-modified gold nanoparticles as a label. Microchimca Acta. 2017;185(1):41.
Kim Young AH, Lee EH, Kim Kwang OK, Lee YT, Hammock BD, Lee HS. Competitive immunochromatographic assay for the detection of the organophosphorus pesticide chlorpyrifos. Anal Chim Acta. 2011;693:106–13.
Xu ZL, Wang Q, Lei HT, Eremin SA, Shen YD, Wang H, et al. A simple, rapid and high-throughput fluorescence polarization immunoassay for simultaneous detection of organophosphorus pesticides in vegetable and environmental water samples. Anal Chim Acta. 2011;708(1–2):123–9.
Sun Z, Wang W, Wen H, Gan C, Lei H, Liu Y. Sensitive electrochemical immunoassay for chlorpyrifos by using flake-like Fe3O4 modified carbon nanotubes as the enhanced multienzyme label. Anal Chim Acta. 2015;899:91–9.
Daems D, Lu J, Delport F, Marien N, Orbie L, Aernouts B, et al. Competitive inhibition assay for the detection of progesterone in dairy milk using a fiber optic SPR biosensor. Anal Chim Acta. 2017;950:1–6.
Shi S, Wang L, Su R, Liu B, Huang R, Qi W, et al. A polydopamine-modified optical fiber SPR biosensor using electroless-plated gold films for immunoassays. Biosens Bioelectron. 2015;74:454–60.
Zhang P, Chen Y-P, Wang W, Shen Y, Guo J-S. Surface plasmon resonance for water pollutant detection and water process analysis. TrAC Trends Anal Chem. 2016;85:153–65.
Gao YM, Zou F, Wu BP, Wang XX, Zhang JJ, Koh K, et al. CB[7]-mediated signal amplification approach for sensitive surface plasmon resonance spectroscopy. Biosens Bioelectron. 2016;81:207–13.
Fen YW, Yunus WM, Talib ZA, Yusof NA. Development of surface plasmon resonance sensor for determining zinc ion using novel active nanolayers as probe. Spectrochim Acta A. 2015;134:48–52.
Masson JF. Surface plasmon resonance clinical biosensors for medical diagnostics. ACS Sens. 2017;2(1):16–30.
Genslein C, Hausler P, Kirchner EM, Bierl R, Baeumner AJ, Hirsch T. Graphene-enhanced plasmonic nanohole arrays for environmental sensing in aqueous samples. Beilstein J Nanotech. 2016;7:1564–73.
Vaisocherova-Lisalova H, Visova I, Ermini ML, Springer T, Song XC, Mrazek J, et al. Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens Bioelectron. 2016;80:84–90.
Liu X, Hu Y, Zheng S, Liu Y, He Z, Luo F. Surface plasmon resonance immunosensor for fast, highly sensitive, and in situ detection of the magnetic nanoparticles-enriched Salmonella enteritidis. Sensors Actuators B Chem. 2016;230:191–8.
Shankaran DR, Gobi KV, Miura N. Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest. Sensors Actuators B Chem. 2007;121(1):158–77.
Feng L, Zhu A, Wang H, Shi H. A nanosensor based on quantum-dot haptens for rapid, on-site immunoassay of cyanotoxin in environmental water. Biosens Bioelectron. 2014;53:1–4.
Li S, Wu Q, Ma P, Zhang Y, Song D, Wang X, et al. A sensitive SPR biosensor based on hollow gold nanospheres and improved sandwich assay with PDA-Ag-Fe3O4-rGO. Talanta. 2018;180:156–61.
Shynkarenko OV, Kravchenko SA. Surface plasmon resonance sensors: methods of surface functionalization and sensitivity enhancement. Theor Exp Chem. 2015;51(5):273–92.
Vashist SK, Dixit CK, MacCraith BD, O’Kennedy R. Effect of antibody immobilization strategies on the analytical performance of a surface plasmon resonance-based immunoassay. Analyst. 2011;136(21):4431–6.
Lu B, Smyth MR, O'Kennedy R. Oriented immobilization of antibodies and its applications in immunoassays and immunosensors. Analyst. 1996;121:29–32.
Mauriz E, Calle A, Lechuga LM, Quintana J, Montoya A, Manclús JJ. Real-time detection of chlorpyrifos at part per trillion levels in ground, surface and drinking water samples by a portable surface plasmon resonance immunosensor. Anal Chim Acta. 2006;561(1–2):40–7.
Thepudom T, Lertvachirapaiboon C, Shinbo K, Kato K, Kaneko F, Kerdcharoen T, Baba A. Surface plasmon resonance-enhanced photoelectrochemical sensor for detection of an organophosphate pesticide chlorpyrifos. MRS Commun. 2017;8(01):107–12.
Acknowledgments
The authors acknowledge Beijing Inter-bio Tech CO., LTD, for providing the surface plasmon resonance device.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81703699, 81573595, and 81603398), the National Project for Standardization of Chinese Materia Medica (No. ZYBZH-Y-JIN-34), CAMS Innovation Fund for Medical Sciences (No. 2017-I2M-1-013), and the Graduate Student Innovation Fund of Peking Union Medical College (No. 2017-1007-14).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Q., Dou, X., Zhang, L. et al. Oriented assembly of surface plasmon resonance biosensor through staphylococcal protein A for the chlorpyrifos detection. Anal Bioanal Chem 411, 6057–6066 (2019). https://doi.org/10.1007/s00216-019-01990-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01990-0