Abstract
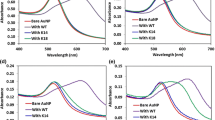
Accumulating findings demonstrate the importance of histone acetyltransferases (HATs) in regulating the acetylation of histones and reveal that their aberrant catalytic activities are involved in the occurrence and progress of numerous diseases. Herein, a feasible electrochemical method is proposed to assay the activity of HAT. The critical elements of the assay method are the hindrance of HAT-catalyzed acetylation against carboxypeptidase Y-catalyzed digestion and cucurbit[8]uril-assisted peptide assembly, which may recruit peptide-templated silver nanoparticles onto the electrode surface, producing significant electrochemical signals. Taking p300 as a model HAT, the assay method is validated to exhibit desirable selectivity, reproducibility, and usability in inhibitor analysis, and allow absolute activity determination in a linear range from 0.1 to 50 nM with a detection limit of 0.055 nM, which is lower than those of previous reports. Therefore, this work may provide an effective tool for HAT activity assay, which will be of great potential in HAT-related fundamental research, disease diagnosis, and drug development in the future.

ᅟ






Similar content being viewed by others
References
Dekker FJ, Haisma HJ. Histone acetyl transferases as emerging drug targets. Drug Discov Today. 2009;14:942–8.
Legube G, Trouche D. Regulating histone acetyltransferases and deacetylases. EMBO Rep. 2003;4:944–7.
Dekker FJ, van den Bosch T, Martin NI. Small molecule inhibitors of histone acetyltransferases and deacetylases are potential drugs for inflammatory diseases. Drug Discov Today. 2014;19:654–60.
Ma F, Zhang CY. Histone modifying enzymes: novel disease biomarkers and assay development. Expert Rev Mol Diagn. 2016;16:297–306.
Zhang K, Dent SY. Histone modifying enzymes and cancer: going beyond histones. J Cell Biochem. 2005;96:1137–48.
Ghadiali JE, Lowe SB, Stevens MM. Quantum-dot-based FRET detection of histone acetyltransferase activity. Angew Chem Int Ed. 2011;50:3417–20.
Han Y, Li P, Xu Y, Li H, Song Z, Nie Z, et al. Fluorescent nanosensor for probing histone acetyltransferase activity based on acetylation protection and magnetic graphitic nanocapsules. Small. 2015;11:877–85.
Chen S, Li Y, Hu Y, Han Y, Huang Y, Nie Z, et al. Nucleic acid-mimicking coordination polymer for label-free fluorescent activity assay of histone acetyltransferases. Chem Commun. 2015;51:4469–72.
He M, Han Z, Qiao J, Ngo L, Xiong M, Zheng Y. A bioorthogonal turn-on fluorescent strategy for the detection of lysine acetyltransferase activity. Chem Commun. 2018;54:5594–7.
He M, Han Z, Liu L, Zheng YG. Chemical biology approaches for investigating the functions of lysine acetyltransferases. Angew Chem Int Ed. 2018;57:1162–84.
Felix FS, Angnes L. Electrochemical immunosensors - a powerful tool for analytical applications. Biosens Bioelectron. 2018;102:470–8.
Shumyantseva VV, Suprun EV, Bulko TV, Archakov AI. Electrochemical methods for detection of post-translational modifications of proteins. Biosens Bioelectron. 2014;61:131–9.
Liu T, Yin J, Wang Y, Miao P. Construction of a specific binding peptide based electrochemical approach for sensitive detection of Zn2+. J Electroanal Chem. 2016;783:304–7.
Jiang Y, Chen XF, Lan LT, Pan Y, Zhu GX, Miao P. Gly–Gly–His tripeptide- and silver nanoparticle-assisted electrochemical evaluation of copper(II) ions in aqueous environment. New J Chem. 2018;42:14733–7.
Li H, Huang Y, Yu Y, Li TQ, Li GX, Anzai J. Enzymatically regulated peptide pairing and catalysis for the bioanalysis of extracellular prometastatic activities of functionally linked enzymes. Sci Rep. 2016;6:25362.
Feng Z, Wang H, Zhou R, Li J, Xu B. Enzyme-instructed assembly and disassembly processes for targeting downregulation in cancer cells. J Am Chem Soc. 2017;139:3950–3.
De Sandis E, Ryadnov MG. Peptide self-assembly for nanomaterials: the old new kid on the block. Chem Soc Rev. 2015;44:8288–300.
Burgess NC, Sharp TH, Thomas F, Wood CW, Thomson AR, Zaccai NR, et al. Modular design of self-assembling peptide-based nanotubes. J Am Chem Soc. 2015;137:10554–62.
Moitra P, Kumar K, Kondaiah P, Bhattacharya S. Efficacious anticancer drug delivery mediated by a pH-sensitive self-assembly of a conserved tripeptide derived from tyrosine kinase NGF receptor. Angew Chem Int Ed. 2014;53:1113–7.
Liu K, Xing RR, Zou QL, Ma GH, Mohwald H, Yan XH. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy. Angew Chem Int Ed. 2016;55:3036–9.
Feng Y, Wang H, Zhang J, Song Y, Meng M, Mi J, et al. Bioinspired synthesis of Au nanostructures templated from amyloid β peptide assembly with enhanced catalytic activity. Biomacromolecules. 2018;19:2432–42.
Wu J, Zou R, Wang Q, Xue Y, Wei P, Yang S, et al. A peptide probe for the detection of neurokinin-1 receptor by disaggregation enhanced fluorescence and magnetic resonance signals. Sci Rep. 2014;4:6487.
Reches M, Gazit E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science. 2003;300:625–7.
Yan XH, Zhu P, Li JB. Self-assembly and application of diphenylalanine-based nanostructures. Chem Soc Rev. 2010;39:1877–90.
Zhao J, Tang Y, Cao Y, Chen T, Chen X, Mao X, et al. Amplified electrochemical detection of surface biomarker in breast cancer stem cell using self-assembled supramolecular nanocomposites. Electrochim Acta. 2018;283:1072–8.
Soum C, Rubio-Albenque S, Fery-Forgues S, Deleris G, Alouini MA, Berthelot T. Supramolecular peptide/surface assembly for monitoring proteinase activity and cancer diagnosis. ACS Appl Mater Interfaces. 2015;7:16967–75.
Chu CW, Ravoo BJ. Hierarchical supramolecular hydrogels: self-assembly by peptides and photo-controlled release via host–guest interaction. Chem Commun. 2017;53:12450–3.
Xue S, Tan C, Chen M, Cao J, Zhang J, Zhang D, et al. Tumor-targeted supramolecular nanoparticles self-assembled from a ruthenium-β-cyclodextrin complex and an adamantane-functionalized peptide. Chem Commun. 2017;53:842–5.
Mao X, Li Y, Han P, Wang X, Yang S, Zhang F, et al. One-pot and one-step colorimetric detection of aminopeptidase N activity based on gold nanoparticles-based supramolecular structure. Sens Actuat B Chem. 2018;267:336–41.
Yan G, Eller MS, Elm C, Larocca CA, Ryu B, Panova IP, et al. Selective inhibition of p300 HAT blocks cell cycle progression, induces cellular senescence, and inhibits the DNA damage response in melanoma cells. J Invest Dermatol. 2013;133:2444–52.
Miao XY, Yu HZ, Gu Z, Yang LL, Teng JH, Cao Y, et al. Peptide self-assembly assisted signal labeling for an electrochemical assay of protease activity. Anal Bioanal Chem. 2017;409:6723–30.
Hu Y, Chen S, Han Y, Chen H, Wang Q, Nie Z, et al. Unique electrocatalytic activity of a nucleic acid-mimicking coordination polymer for the sensitive detection of coenzyme A and histone acetyltransferase activity. Chem Commun. 2015;51:17611–4.
Clements A, Poux AN, Lo WS, Pillus L, Berger SL, Marmorstein R. Structural basis for histone and phosphohistone binding by the GCN5 histone acetyltransferase. Mol Cell. 2003;12:461–73.
Han Z, Chou CW, Yang X, Bartlett MG, Zheng YG. Profiling cellular substrates of lysine acetyltransferases GCN5 and p300 with orthogonal labeling and click chemistry. ACS Chem Biol. 2017;12:1547–55.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81503463), the Science and Technology Planning Project of Taicang (TC2018JC04) and the Natural Science Research Projects of Universities in Jiangsu Province (Project No. 16KJB430037).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 240 kb)
Rights and permissions
About this article
Cite this article
Miao, X., Wang, Y., Gu, Z. et al. Cucurbit[8]uril-assisted peptide assembly for feasible electrochemical assay of histone acetyltransferase activity. Anal Bioanal Chem 411, 387–393 (2019). https://doi.org/10.1007/s00216-018-1445-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1445-4