Abstract
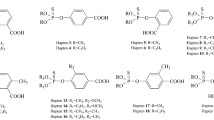
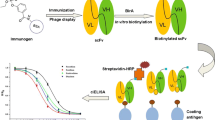
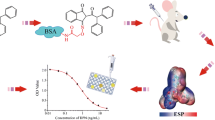
In this study, heterologous indirect competitive enzyme-linked immunosorbent assay (icELISA) was introduced into the screening of hybridomas for the development of broad-specific monoclonal antibodies (mAbs) against organophosphorus (OP) pesticides. After immunization, two formats of icELISA based on the homologous hapten antigen and four heterologous hapten antigens were conducted for hybridoma screening. Two mAbs 2G6 and 7B2 with good recognition toward three OP pesticides (parathion, methyl-parathion, and fenitrothion) were produced. Results of the icELISA showed that the two mAbs exhibited high sensitivity against three OP pesticides, with IC50 ranging from 2.93 to 19.71 ng mL−1. Moreover, a non-competitive surface plasmon resonance (SPR) immunosensor was used for characterizing the binding properties of the mAbs to OP pesticides. After kinetic analysis, equilibrium dissociation constant (KD) values of mAbs 2G6 and 7B2 were calculated as 1.45 × 10−9 M and 4.26 × 10−9 M for parathion, 6.75 × 10−9 M and 4.17 × 10−9 M for methyl-parathion, and 2.44 × 10−8 M and 1.19 × 10−8 M for fenitrothion, respectively. Whereas, both icELISA and SPR-based immunoassay indicated that the two mAbs could not recognize other five OP analogs. Since SPR-based immunoassay provides comprehensive information of two molecules directly interacting with each other, it is a valuable tool during the development and characterization of broad-specific mAbs.

ᅟ




Similar content being viewed by others
References
Roex EWM, Keijzers R, van Gestel CAM. Acetylcholinesterase inhibition and increased food consumption rate in the zebrafish, Danio rerio, after chronic exposure to parathion. Aquat Toxicol. 2003;64:451–60.
Zhao X, Kong W, Wei J, Yang M. Gas chromatography with flame photometric detection of 31 organophosphorus pesticide residues in Alpinia oxyphylla dried fruits. Food Chem. 2014;162:270–6.
Harshit D, Charmy K, Nrupesh P. Organophosphorus pesticides determination by novel HPLC and spectrophotometric method. Food Chem. 2017;230:448–53.
Andrade GCRM, Monteiro SH, Francisco JG, Figueiredo LA, Botelho RG, Tornisielo VL. Liquid chromatography–electrospray ionization tandem mass spectrometry and dynamic multiple reaction monitoring method for determining multiple pesticide residues in tomato. Food Chem. 2015;175:57–65.
Liu Y, Liu R, Boroduleva A, Eremin S, Guo Y, Zhu G. A highly specific and sensitive fluorescence polarization immunoassay for the rapid detection of triazophos residue in agricultural products. Anal Methods. 2016;8:6636–44.
Lan M, Guo Y, Zhao Y, Liu Y, Gui W, Zhu G. Multi-residue detection of pesticides using a sensitive immunochip assay based on nanogold enhancement. Anal Chim Acta. 2016;938:146–55.
Zhao F, Hu C, Wang H, Zhao L, Yang Z. Development of a MAb-based immunoassay for the simultaneous determination of O,O-diethyl and O,O-dimethyl organophosphorus pesticides in vegetable and fruit samples pretreated with QuEChERS. Anal Bioanal Chem. 2015;407:8959–70.
Xu Z, Sun W, Yang J, Jiang Y, Campbell K, Shen Y, et al. Development of a solid-phase extraction coupling chemiluminescent enzyme immunoassay for determination of organophosphorus pesticides in environmental water samples. J Agric Food Chem. 2012;60:2069–75.
Du P, Jin M, Yang L, Du X, Chen G, Zhang C, et al. A rapid immunomagnetic-bead-based immunoassay for triazophos analysis. RSC Adv. 2015;5:81046–51.
Shi H, Li H, Hua X, Zheng Z, Zhu G, Wang M. Characterization of multihapten antigens on antibody sensitivity and specificity for parathion. Anal Lett. 2014;47:2699–707.
Xu Z, Xie G, Li Y, Wang B, Beier RC, Lei H, et al. Production and characterization of a broad-specificity polyclonal antibody for O,O-diethyl organophosphorus pesticides and a quantitative structure–activity relationship study of antibody recognition. Anal Chim Acta. 2009;647:90–6.
Liu B, Ge Y, Zhang Y, Song Y, Lv Y, Wang X, et al. Production of the class-specific antibody and development of direct competitive ELISA for multi-residue detection of organophosphorus pesticides. Food Agric Immunol. 2012;23:157–68.
Piao YZ, Kim YJ, Kim YA, Lee H, Hammock BD, Lee YT. Development of ELISAs for the class-specific determination of organophosphorus pesticides. J Agric Food Chem. 2009;57:10004–13.
Wang C, Li X, Liu Y, Guo Y, Xie R, Gui W, et al. Development of a Mab-based heterologous immunoassay for the broad-selective determination of organophosphorus pesticides. J Agric Food Chem. 2010;58:5658–63.
Wang Z, Li N, Zhang S, Zhang H, Sheng Y, Shen J. Production of antibodies and development of enzyme-linked immunosorbent assay for valnemulin in porcine liver. Food Addit Contam: Part A. 2013;30:244–52.
Guo Y, Sanders M, Galvita A, Heyerick A, Deforce D, Bracke M, et al. Heterologous screening of hybridomas for the development of broad-specific monoclonal antibodies against deoxynivalenol and its analogues. World Mycotoxin J. 2014;7:257–65.
Chang AL, McKeague M, Liang JC, Smolke CD. Kinetic and equilibrium binding characterization of aptamers to small molecules using a label-free, sensitive, and scalable platform. Anal Chem. 2014;86:3273–8.
Scarano S, Mascini M, Turner APF, Minunni M. Surface plasmon resonance imaging for affinity-based biosensors. Biosens Bioelectron. 2010;25:957–66.
Estevez MC, Belenguer J, Gomez-Montes S, Miralles J, Escuela AM, Montoya A, et al. Indirect competitive immunoassay for the detection of fungicide thiabendazole in whole orange samples by surface plasmon resonance. Analyst. 2012;137:5659–65.
Mauriz E, García-Fernández C, Mercader JV, Abad-Fuentes A, Escuela AM, Lechuga LM. Direct surface plasmon resonance immunosensing of pyraclostrobin residues in untreated fruit juices. Anal Bioanal Chem. 2012;404:2877–86.
Hirakawa Y, Yamasaki T, Harada A, Ohtake T, Adachi K, Iwasa S, et al. Analysis of the fungicide boscalid in horticultural crops using an enzyme-linked immunosorbent assay and an immunosensor based on surface plasmon resonance. J Agric Food Chem. 2015;63:8075–82.
Yakes BJ, Kanyuck KM, DeGrasse SL. First report of a direct surface plasmon resonance immunosensor for a small molecule seafood toxin. Anal Chem. 2014;86:9251–5.
Guo Y, Liu R, Liu Y, Xiang D, Liu Y, Gui W, et al. A non-competitive surface plasmon resonance immunosensor for rapid detection of triazophos residue in environmental and agricultural samples. Sci Total Environ. 2018;613-614:783–91.
Langer N, Steinicke F, Lindigkeit R, Ernst L, Beuerle T. Determination of cross-reactivity of poly- and monoclonal antibodies for synthetic cannabinoids by direct SPR and ELISA. Forensic Sci Int. 2017;280:25–34.
Liu Y, Jin M, Gui W, Cheng J, Guo Y, Zhu G. Hapten design and indirect competitive immunoassay for parathion determination: correlation with molecular modeling and principal component analysis. Anal Chim Acta. 2007;591:173–82.
McAdam DP, Hill AS, Beasley HL, Skerritt JH. Mono- and polyclonal antibodies to the organophosphate fenitrothion. 1. Approaches to hapten–protein conjugation. J Agric Food Chem. 1992;40:1466–70.
Riggle B, Dunbar B. Development of enzyme immunoassay for the detection of the herbicide norflurazon. J Agric Food Chem. 1990;38:1922–5.
Wang C, Liu Y, Guo Y, Liang C, Li X, Zhu G. Development of a McAb-based immunoassay for parathion and influence of the competitor structure. Food Chem. 2009;115:365–70.
Cannon MJ, Papalia GA, Navratilova I, et al. Comparative analyses of a small molecule/enzyme interaction by multiple users of Biacore technology. Anal Biochem. 2004;330:98–113.
Henniona M, Barcelo D. Strengths and limitations of immunoassays for effective and efficient use for pesticide analysis in water samples: a review. Anal Chim Acta. 1998;362:3–34.
Kim YJ, Cho YA, Lee H, Lee YT. Investigation of the effect of hapten heterology on immunoassay sensitivity and development of an enzyme-linked immunosorbent assay for the organophosphorus insecticide fenthion. Anal Chim Acta. 2003;494:29–40.
Holthues H, Pfeifer-Fukumura U, Sound I, Baumann W. Evaluation of the concept of heterology in a monoclonal antibody-based ELISA utilizing direct hapten linkage to polystyrene microtiter plates. J Immunol Methods. 2005;304:68–77.
Dong S, Zhang C, Zhang X, Liu Y, Zhong J, Xie Y, et al. Production and characterization of monoclonal antibody broadly recognizing Cry1 toxins by use of designed polypeptide as Hapten. Anal Chem. 2016;88:7023–32.
Li X, Zhang H, Ji Y, Zheng Z, Bian Q, Zhu G. Immunochemical and molecular characteristics of monoclonal antibodies against organophosphorus pesticides and effect of hapten structures on immunoassay selectivity. Food Agric Immunol. 2013;26:109–19.
Acknowledgements
This study was financially supported by National Key R&D Program of China (2017YFF0210200 and 2016YFD0201302) and the Agricultural Project for Public Technology Research in Zhejiang Province (2016C32004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All studies on mice were performed under the guidance of the animal welfare committee of Zhejiang University in China.
Conflict of interests
The authors have declared no conflict of interests.
Electronic supplementary material
ESM 1
(PDF 175 kb)
Rights and permissions
About this article
Cite this article
Jiao, S., Liu, P., Liu, Y. et al. Binding properties of broad-specific monoclonal antibodies against three organophosphorus pesticides by a direct surface plasmon resonance immunosensor. Anal Bioanal Chem 410, 7263–7273 (2018). https://doi.org/10.1007/s00216-018-1337-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1337-7