Abstract
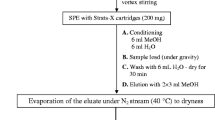
The scarcity of data about the occurrence of pharmaceuticals in water bodies in Malaysia prompted us to develop a suitable analytical method to address this issue. We therefore developed a method based on solid-phase extraction combined with liquid chromatography–time of flight/mass spectrometry (SPE-LC-TOF/MS) for the analysis of sixteen prescribed and two nonprescribed pharmaceuticals that are potentially present in water samples. The levels of these pharmaceuticals, which were among the top 50 pharmaceuticals consumed in Malaysia during the period 2011–2014, in influent and effluent of five sewage treatment plants (STPs) in Bangi, Malaysia, were then analyzed using the developed method. All of the pharmaceuticals were separated chromatographically using a 5 μm, 2.1 mm × 250 mm C18 column at a flow rate of 0.3 mL/min. Limits of quantification (LOQs) were 0.3–8.2 ng/L, 6.5–89 ng/L, and 11.1–93.8 ng/L in deionized water (DIW), STP effluent, and STP influent, respectively, for most of the pharmaceuticals. Recoveries were 51–108%, 52–118%, and 80–107% from the STP influent, STP effluent, and DIW, respectively, for most of the pharmaceuticals. The matrix effect was also evaluated. The signals from carbamazepine, diclofenac sodium, and mefenamic acid were found to be completely suppressed in the STP influent. The signals from other compounds were found to be influenced by matrix effects more strongly in STP influent (enhancement or suppression of signal ≤180%) than in effluent (≤94%). The signal from prednisolone was greatly enhanced in the STP influent, indicating a matrix effect of −134%. Twelve pharmaceuticals were frequently detected in all five STPs, and caffeine, prazosin, and theophylline presented the highest concentrations among all the pharmaceuticals monitored: up to 7611, 550, and 319 ng/L in the STP influent, respectively. To the best of our knowledge, this is the first time that prazosin has been detected in a water matrix in Malaysia.

ᅟ







Similar content being viewed by others
References
Ministry of Health Malaysia. Malaysian statistics on medicine. 2014. http://apps.who.int/medicinedocs/documents/s17580en/s17580en.pdf. Accessed 20 February 2018.
García SO, García-Encina PA, Irusta-Mata R. The potential ecotoxicological impact of pharmaceutical and personal care products on humans and freshwater, based on USEtox™ characterization factors. A Spanish case study of toxicity impact scores. Sci Total Environ. 2017;609:429–45.
WHO, editor. Guidelines for drinking-water quality. 4th ed. Geneva: World Health Organization; 2011.
Schwaiger J, Ferling H, Mallow U, Wintermayr H, Negele RD. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: part I: histopathological alterations and bioaccumulation in rainbow trout. Aquat Toxicol. 2004;68:141–50.
McEneff G, Barron L, Kelleher B, Paull B, Quinn B. A year-long study of the spatial occurrence and relative distribution of pharmaceutical residues in sewage effluent, receiving marine waters and marine bivalves. Sci Total Environ. 2014;476:317–26.
El Khatib MS, Koubar M, Daher M, Z. Al Iskandarani M. Innovative SPE-LC-MS/MS technique for the assessment of 63 pharmaceuticals and the detection of antibiotic-resistant-bacteria: a case study natural water sources in Lebanon. Sci Total Environ. 2017;609:830–41.
Ferrer I, Thurman EM. Analysis of 100 pharmaceuticals and their degradates in water samples by liquid chromatography/quadrupole time-of-flight mass spectrometry. J Chromatogr A. 2012;1259:148–57.
Unceta N, Sampedro MC, Bakar NK, Gómez-Caballero A, Goicolea MA, Barrio RJ. Multi-residue analysis of pharmaceutical compounds in wastewaters by dual solid-phase microextraction coupled to liquid chromatography electrospray ionization ion trap mass spectrometry. J Chromatogr A. 2010;1217:3392–9.
Bicker J, Fortuna A, Alves G, Falcão A. Liquid chromatographic methods for the quantification of catecholamines and their metabolites in several biological samples—a review. Anal Chim Acta. 2013;768:12–34.
Jiang L, Chen Y, Chen Y, Ma M, Tan Y, Tang H, et al. Determination of monoamine neurotransmitters in human urine by carrier-mediated liquid-phase microextraction based on solidification of stripping phase. Talanta. 2015;144:356–62.
Paíga P, Lolić A, Hellebuyck F, Santos LH, Correia M, Delerue-Matos C. Development of a SPE–UHPLC–MS/MS methodology for the determination of non-steroidal anti-inflammatory and analgesic pharmaceuticals in seawater. J Pharmaceut Biomed. 2015;106:61–70.
Petrović M, Škrbić B, Živančev J, Ferrando-Climent L, Barcelo D. Determination of 81 pharmaceutical drugs by high performance liquid chromatography coupled to mass spectrometry with hybrid triple quadrupole–linear ion trap in different types of water in Serbia. Sci Total Environ. 2014;468:415–28.
Al-Qaim FF, Abdullah MP, Othman MR, Latip J, Afiq W. A validation method development for simultaneous LC-ESI-TOF/MS analysis of some pharmaceuticals in Tangkas River—Malaysia. J Brazil Chem Soc. 2014;25:271–81.
Rogers HR. Sources, behaviour and fate of organic contaminants during sewage treatment and in sewage sludges. Sci Total Environ. 1996;185:3–26.
Nabil AH, Saad B, Grote M. A solid bar microextraction method for the liquid chromatographic determination of trace diclofenac, ibuprofen and carbamazepine in river water. Microchim Acta. 2011;172:31–7.
Ascar L, Ahumada I, López A, Quintanilla F, Leiva K. Nonsteroidal anti-inflammatory drug determination in water samples by HPLC-DAD under isocratic conditions. J Brazil Chem Soc. 2013;24:1160–6.
Peysson W, Vulliet E. Determination of 136 pharmaceuticals and hormones in sewage sludge using quick, easy, cheap, effective, rugged and safe extraction followed by analysis with liquid chromatography–time-of-flight-mass spectrometry. J Chromatogr A. 2013;1290:46–61.
Stolker AA, Niesing W, Hogendoorn EA, Versteegh JF, Fuchs R, Udo AT. Liquid chromatography with triple-quadrupole or quadrupole-time of flight mass spectrometry for screening and confirmation of residues of pharmaceuticals in water. Anal Bioanal Chem. 2004;378:955–63.
Bollmann AF, Seitz W, Prasse C, Lucke T, Schulz W, Ternes T. Occurrence and fate of amisulpride, sulpiride, and lamotrigine in municipal wastewater treatment plants with biological treatment and ozonation. J Hazard Mater. 2016;320:204–15.
American Public Health Association. Standard methods for the examination of water and wastewater. 21st ed. Washington, DC: APHA; 2005.
Vieno NM, Tuhkanen T, Kronberg L. Analysis of neutral and basic pharmaceuticals in sewage treatment plants and in recipient rivers using solid phase extraction and liquid chromatography–tandem mass spectrometry detection. J Chromatogr A. 2006;1134:101–11.
Petrovic M, Gros M, Barcelo D. Multi-residue analysis of pharmaceuticals in wastewater by ultra-performance liquid chromatography–quadrupole–time-of-flight mass spectrometry. J Chromatogr A. 2006;1124:68–81.
Martín J, Buchberger W, Alonso E, Himmelsbach M, Aparicio I. Comparison of different extraction methods for the determination of statin drugs in wastewater and river water by HPLC/Q-TOF-MS. Talanta. 2011;85:607–15.
Hernando MD, Petrovic M, Fernández-Alba AR, Barceló D. Analysis by liquid chromatography–electrospray ionization tandem mass spectrometry and acute toxicity evaluation for β-blockers and lipid-regulating agents in wastewater samples. J Chromatogr A. 2004;1046:133–40.
Gros M, Petrović M, Barceló D. Development of a multi-residue analytical methodology based on liquid chromatography–tandem mass spectrometry (LC–MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta. 2006;70:678–90.
Ferrer I, Zweigenbaum JA, Thurman EM. Analysis of 70 Environmental Protection Agency priority pharmaceuticals in water by EPA method 1694. J Chromatogr A. 2010;1217:5674–86.
Shaaban H, Górecki T. Fast ultrahigh performance liquid chromatographic method for the simultaneous determination of 25 emerging contaminants in surface water and wastewater samples using superficially porous sub-3 μm particles as an alternative to fully porous sub-2 μm particles. Talanta. 2012;100:80–9.
Pedrouzo M, Borrull F, Pocurull E, Marcé RM. Presence of pharmaceuticals and hormones in waters from sewage treatment plants. Water Air Soil Pollut. 2011;217:267–81.
Huggett DB, Khan IA, Foran CM, Schlenk D. Determination of beta-adrenergic receptor blocking pharmaceuticals in United States wastewater effluent. Environ Pollut. 2003;121:199–205.
Ternes T, Bonerz M, Schmidt T. Determination of neutral pharmaceuticals in wastewater and rivers by liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr A. 2001;938:175–85.
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. Multiresidue methods for the analysis of pharmaceuticals, personal care products and illicit drugs in surface water and wastewater by solid-phase extraction and ultra performance liquid chromatography–electrospray tandem mass spectrometry. Anal Bioanal Chem. 2008;391:1293–308.
Kim JW, Jang HS, Kim JG, Ishibashi H, Hirano M, Nasu K, et al. Occurrence of pharmaceutical and personal care products (PPCPs) in surface water from Mankyung River. South Korea J Health Sci. 2009;55:249–58.
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. Illicit drugs and pharmaceuticals in the environment—forensic applications of environmental data, part 2: pharmaceuticals as chemical markers of faecal water contamination. Environ Pollut. 2009;157:1778–86.
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales. UK Water Res. 2008;42:3498–518.
Khetan SK, Collins TJ. Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chem Rev. 2007;107:2319–64.
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. Multi-residue method for the determination of basic/neutral pharmaceuticals and illicit drugs in surface water by solid-phase extraction and ultra performance liquid chromatography–positive electrospray ionization tandem mass spectrometry. J Chromatogr A. 2007;1161:132–45.
Miao XS, Metcalfe CD. Determination of cholesterol-lowering statin drugs in aqueous samples using liquid chromatography–electrospray ionization tandem mass spectrometry. J Chromatogr A. 2003;998:133–41.
Vanderford BJ, Snyder SA. Analysis of pharmaceuticals in water by isotope dilution liquid chromatography/tandem mass spectrometry. Environ Sci Technol. 2006;40:7312–20.
Rivera-Jaimes JA, Postigo C, Melgoza-Alemán RM, Aceña J, Barceló D, de Alda ML. Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: occurrence and environmental risk assessment. Sci Total Environ. 2018;613:1263–74.
Félix-Cañedo TE, Durán-Álvarez JC, Jiménez-Cisneros B. 2013. The occurrence and distribution of a group of organic micropollutants in Mexico City's water sources. Sci Total Environ. 2013;454:109–18.
Biel-Maeso M, Baena-Nogueras RM, Corada-Fernández C, Lara-Martín PA. Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Sci Total Environ. 2018;612:649–59.
Acknowledgements
The authors are grateful to the MJIIT-UTM for giving us the opportunity to conduct and report this research work, and for their support. The authors also thank Central Research for Instrumental Management (CRIM) for supplying us with all the instrumentation we needed to do this work. In addition, the authors are grateful for the technical support of the Indah Water Company during the sampling campaigns.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 329 kb)
Rights and permissions
About this article
Cite this article
Al-Qaim, F.F., Mussa, Z.H. & Yuzir, A. Development and validation of a comprehensive solid-phase extraction method followed by LC-TOF/MS for the analysis of eighteen pharmaceuticals in influent and effluent of sewage treatment plants. Anal Bioanal Chem 410, 4829–4846 (2018). https://doi.org/10.1007/s00216-018-1120-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1120-9