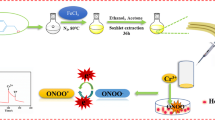
Abstract
A high sensitive and selective hydrogen peroxide (H2O2) biosensor was fabricated on the basis of reduced hemoglobin (Hb) and single-walled carbon nanotubes (SWCNTs) for detecting the release of H2O2 from living HepG2 cancer cells in the process of the in situ biosynthesis of ZnO quantum. The modification of carbon fiber microelectrode (CFME) was carried out by physical adsorption. By the scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS), the dense cover of surface and successful immobilization were characterized. Electrochemical investigation demonstrates that the as-prepared modified microelectrode showed a quasi-reversible process toward the reduction of H2O2, which exhibited a linear range from 0.51 to 10.6 μM, with a limit of detection of 0.23 μM. This microelectrode biosensor was applied for the quantification of the change of H2O2 concentration released from HepG2 cells through the in situ biosynthesis of ZnO quantum dots, which was further confirmed by the fluorescence staining.









Similar content being viewed by others
Change history
05 July 2018
The authors would like to call the reader’s attention to the fact that unfortunately Alberto Pasquarelli’s and Kay-Eberhard Gottschalk’s affiliations were wrong in the original publication.
References
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84.
Chang H, Wang X, Shiu K-K, Zhu Y, Wang J, Li Q, et al. Layer-by-layer assembly of graphene, au and poly (toluidine blue O) films sensor for evaluation of oxidative stress of tumor cells elicited by hydrogen peroxide. Biosens Bioelectron. 2013;41:789–94.
Roberts JG, Hamilton KL, Sombers LA. Comparison of electrode materials for the detection of rapid hydrogen peroxide fluctuations using background-subtracted fast scan cyclic voltammetry. Analyst. 2011;136(17):3550–6.
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266(1–2):37–56.
Behl C, Davis J, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid Β protein toxicity. Cell. 1994;77(6):817–27.
Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer's disease. J Alzheimers Dis. 2010;19(1):341–53.
Weisskopf M, O'reilly E, Chen H, Schwarzschild M, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol. 2007;166(5):561–7.
Brieger K, Schiavone S, Miller FJ Jr, Krause K-H. Reactive oxygen species: from health to disease. Swiss Med Wkly. 2012;142:w13659.
Pryor WA. Vitamin E and heart disease: basic science to clinical intervention trials. Free Radic Biol Med. 2000;28(1):141–64.
Miller EW, Chang CJ. Fluorescent probes for nitric oxide and hydrogen peroxide in cell signaling. Curr Opin Chem Biol. 2007;11(6):620–5.
Paulsen CE, Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2009;5(1):47–62.
Matés JM, Segura JA, Alonso FJ, Márquez J. Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol. 2008;82(5):273–99.
Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci. 2010;107(36):15681–6.
Yao S, Xu J, Wang Y, Chen X, Xu Y, Hu S. A highly sensitive hydrogen peroxide Amperometric sensor based on MnO2 nanoparticles and Dihexadecyl hydrogen phosphate composite film. Anal Chim Acta. 2006;557(1–2):78–84.
Wang K, Liu Q, Wu X-Y, Guan Q-M, Li H-N. Graphene enhanced electrochemiluminescence of Cds nanocrystal for H2O2 sensing. Talanta. 2010;82(1):372–6.
Wen F, Dong Y, Feng L, Wang S, Zhang S, Zhang X. Horseradish peroxidase functionalized fluorescent gold nanoclusters for hydrogen peroxide sensing. Anal Chem. 2011;83(4):1193–6.
Nogueira RFP, Oliveira MC, Paterlini WC. Simple and fast spectrophotometric determination of H2O2 in photo-Fenton reactions using Metavanadate. Talanta. 2005;66(1):86–91.
Wightman RM. Probing cellular chemistry in biological systems with microelectrodes. Science. 2006;311(5767):1570–4.
Forster RJ. Microelectrodes: new dimensions in electrochemistry. Chem Soc Rev. 1994;23(4):289–97.
Huffman ML, Venton BJ. Carbon-Fiber microelectrodes for in vivo applications. Analyst. 2009;134(1):18–24.
Sanford AL, Morton SW, Whitehouse KL, Oara HM, Lugo-Morales LZ, Roberts JG, et al. Voltammetric detection of hydrogen peroxide at carbon fiber microelectrodes. Anal Chem. 2010;82(12):5205–10.
Wu Z, Chen L, Shen G, Yu R. Platinum nanoparticle-modified carbon fiber ultramicroelectrodes for mediator-free biosensing. Sensors Actuators B Chem. 2006;119(1):295–301.
Ross AE, Venton BJ. Nafion–Cnt coated carbon-Fiber microelectrodes for enhanced detection of adenosine. Analyst. 2012;137(13):3045–51.
Wen Z, Ci S, Li J. Pt nanoparticles inserting in carbon nanotube arrays: nanocomposites for glucose biosensors. J Phys Chem C. 2009;113(31):13482–7.
Zhang Y, Bai X, Wang X, Shiu K-K, Zhu Y, Jiang H. Highly sensitive graphene–Pt nanocomposites Amperometric biosensor and its application in living cell H2O2 detection. Anal Chem. 2014;86(19):9459–65.
Li C, Liu X, Zhang Y, Chen Y, Du T, Jiang H, et al. A novel nonenzymatic biosensor for evaluation of oxidative stress based on nanocomposites of graphene blended with cui. Anal Chim Acta. 2016;933:66–74.
Li Y, Zhang J-J, Xuan J, Jiang L-P, Zhu J-J. Fabrication of a novel nonenzymatic hydrogen peroxide sensor based on Se/Pt nanocomposites. Electrochem Commun. 2010;12(6):777–80.
Kafi A, Yin F, Shin H-K, Kwon Y-S. Hydrogen peroxide biosensor based on DNA–Hb modified gold electrode. Thin Solid Films. 2006;499(1–2):420–4.
Patolsky F, Weizmann Y, Willner I. Long-range electrical contacting of redox enzymes by Swcnt connectors. Angew Chem Int Ed. 2004;43(16):2113–7.
Guiseppi-Elie A, Lei C, Baughman RH. Direct Electron transfer of glucose oxidase on carbon nanotubes. Nanotechnology. 2002;13(5):559.
Yao Y, Shiu K-K. Electron-transfer properties of different carbon nanotube materials, and their use in glucose biosensors. Anal Bioanal Chem. 2007;387(1):303–9.
Yagati AK, Choi JW. Protein based electrochemical biosensors for H2o2 detection towards clinical diagnostics. Electroanalysis. 2014;26(6):1259–76.
Ren QQ, Yuan XJ, Huang XR, Wen W, Zhao YD, Chen W. In vivo monitoring of oxidative burst on Aloe under salinity stress using hemoglobin and single-walled carbon nanotubes modified carbon fiber ultramicroelectrode. Biosens Bioelectron. 2013;50:318–24.
Chen W, Cai S, Ren QQ, Wen W, Zhao Y-D. Recent advances in electrochemical sensing for hydrogen peroxide: a review. Analyst. 2012;137(1):49–58.
Du T, Zhao C, ur Rehman F, Lai L, Li X, Sun Y, et al. Rapid and multimodal in vivo bioimaging of cancer cells through in situ biosynthesis of Zn&Fe nanoclusters. Nano Res. 2017;10(8):2626–32.
Simizu S, Takada M, Umezawa K, Imoto M. Requirement of caspase-3 (-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem. 1998;273(41):26900–7.
Fan Y, Yang H, Liu X, Zhu H, Zou G. Preparation and study on radar absorbing materials of nickel-coated carbon fiber and flake graphite. J Alloys Compd. 2008;461(1):490–4.
Song S, Dong S. Spectroelectrochemistry of the quasi-reversible reduction and oxidation of hemoglobin at a methylene blue adsorbed modified electrode. J Electroanal Chem Interfac. 1988;253(2):337–46.
Yan Y, Zheng W, Zhang M, Wang L, Su L, Mao L. Bioelectrochemically functional Nanohybrids through co-assembling of proteins and surfactants onto carbon nanotubes: facilitated electron transfer of assembled proteins with enhanced faradic response. Langmuir. 2005;21(14):6560–6.
Lu Q, Hu S, Pang D, He Z. Direct electrochemistry and electrocatalysis with hemoglobin in water-soluble quantum dots film on glassy carbon electrode. Chem Commun. 2005;20:2584–5.
Meites L, Delahay P. Polarographic techniques. J Electrochem Soc. 1966;113(5):124C.
Zhang XW, Qiu QF, Jiang H, Zhang FL, Liu YL, Amatore C, et al. Real-time intracellular measurements of Ros and Rns in living cells with single core–shell nanowire electrodes. Angew Chem Int Ed. 2017;56(42):12997–3000.
Kamin RA, Wilson GS. Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal Chem. 1980;52(8):1198–205.
Sun W, Rf G, Jiao K. Electrochemistry and electrocatalysis of a Nafion/Nano-Caco3/Hb film modified carbon ionic liquid electrode using Bmimpf6 as binder. Electroanalysis. 2007;19(13):1368–74.
Wang H, Guan R, Fan C, Zhu D, Li G. A hydrogen peroxide biosensor based on the bioelectrocatalysis of hemoglobin incorporated in a Kieselgubr film. Sensors Actuators B Chem. 2002;84(2–3):214–8.
He R, Tang H, Jiang D. Chen H-y. Electrochemical visualization of intracellular hydrogen peroxide at single cells. Anal Chem. 2016;88(4):2006–9.
Davey MW, Montagu MV, Inzé D, Sanmartin M, Kanellis A, Smirnoff N, et al. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric. 2000;80(7):825–60.
Chen J, He Z, Liu H, Cha C. Electrochemical determination of reduced glutathione (Gsh) by applying the powder microelectrode technique. J Electroanal Chem. 2006;588(2):324–30.
Jones R, Morice A. Hydrogen peroxide—an intracellular signal in the pulmonary circulation: involvement in hypoxic pulmonary vasoconstriction. Pharmacol Ther. 2000;88(2):153–61.
Gorman A, McGowan A, Cotter TG. Role of peroxide and superoxide anion during tumour cell apoptosis. FEBS Lett. 1997;404(1):27–33.
Enami S, Sakamoto Y, Colussi AJ. Fenton chemistry at aqueous interfaces. Proc Natl Acad Sci. 2014;111(2):623–8.
Acknowledgements
This work is supported by the National High-tech R&D Program and National Key Research & Development Program of China (Nos. 2017YFA0205301), and the National Natural Science Foundation of China (Nos. 91753106, 81325011, 21327902).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, H., Ruan, J., Liu, W. et al. Monitoring dynamic release of intracellular hydrogen peroxide through a microelectrode based enzymatic biosensor. Anal Bioanal Chem 410, 4509–4517 (2018). https://doi.org/10.1007/s00216-018-1108-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1108-5