Abstract
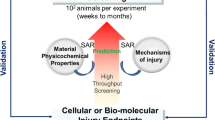
Nanomaterials (NMs) are widely used in various areas because of their unique and useful physicochemical properties. However, they may pose toxicity risks to human health after exposure. Applicable and reliable approaches are needed for risk assessment of NMs. Herein, an intelligent analytical strategy for safety assessment of NMs is proposed that focuses on toxicity assessment using an in vitro cell model. The toxicity assessment by testing on the adverse outcome pathway in a cell culture system was defined by application of a tiered testing approach. To provide an overview of the applicable approach for risk assessment of NMs, we discuss the most commonly used techniques and analytical methods, including computational toxicology methods in dosimetry assessment, high-throughput screening for toxicity testing with high efficiency, and omics-based toxicology assessment methods. The final section focuses on the route map for an integrated approach to a testing and assessment strategy on how to extrapolate the in vitro NM toxicity testing data to in vivo risk assessment of NMs. The intelligent analytical strategy, having evolved step-by-step, could contribute to better applications for safety evaluation and risk assessment of NMs in reality.




Similar content being viewed by others
References
Bhushan B, editor. Springer handbook of nanotechnology. 4th ed. Berlin: Springer; 2017.
Du JF, Zhang X, Yan L, Chen R. Functional tumor imaging based on inorganic nanomaterials. Sci China Chem. 2017;60(11):1425–38.
Chen R, Chen C. Environment, health and safety issues in nanotechnology. In: Bhushan B, editor. Springer handbook of nanotechnology. 4th ed. Berlin: Springer; 2017. p. 1503–24.
Chen R, Chen C. Exposure scenarios in the workplace and risk assessment of carbon nanomaterials. In: Chen C, Wang H, editors. Biomedical applications and toxicology of carbon nanomaterials. Weinheim: Wiley-VCH; 2016. p. 515–34.
Wang J, Quershi WA, Li YY, Xu JX, Nie GJ. Analytical methods for nano-bio interface interactions. Sci China Chem. 2016;59(11):1467–78.
Chen R, Chen CY. Nanotoxicity. In: Xie YB, editor. The nanobiotechnology handbook. Abingdon: Taylor & Francis; 2012. p. 599–620.
Chen C, Li YF. Qu Y, Chai Z, Zhao Y. Advanced nuclear analytical and related techniques for the growing challenges in nanotoxicology. Chem Soc Rev. 2013;42(21):8266–303.
Wang B, Wang Z, Feng W, Wang M. Hu Z, Chai Z, et al. New methods for nanotoxicology: synchrotron radiation-based techniques. Anal Bioanal Chem. 2010;398(2):667–76.
Li YF, Zhao J, Qu Y, Gao Y, Guo Z, Liu Z, et al. Synchrotron radiation techniques for nanotoxicology. Nanomedicine. 2015;11(6):1531–49.
OECD. Test no. 413: subchronic inhalation toxicity: 90-day study. Paris: OECD Publishing; 2009.
OECD. Test no. 412: subacute inhalation toxicity: 28-day study. Paris: OECD Publishing; 2009.
OECD. Test no. 403: acute inhalation toxicity. Paris: OECD Publishing; 2009.
Nel A, Xia T, Meng H, Wang X, Lin S, Ji Z, et al. Nanomaterial toxicity testing in the 21st century: use of a predictive toxicological approach and high-throughput screening. Acc Chem Res. 2013;46(3):607–21.
Farcal L, Torres Andon F, Di Cristo L, Rotoli BM, Bussolati O, Bergamaschi E, et al. Comprehensive in vitro toxicity testing of a panel of representative oxide nanomaterials: first steps towards an intelligent testing strategy. PLoS One. 2015;10(5):e0127174.
Firestone M, Kavlock R, Zenick H, Kramer M. The U.S. Environmental Protection Agency strategic plan for evaluating the toxicity of chemicals. J Toxicol Environ Health B Crit Rev. 2010;13(2-4):139–62.
Krewski D, Acosta D Jr, Andersen M, Anderson H, Bailar JC 3rd, Boekelheide K, et al. Toxicity testing in the 21st century: a vision and a strategy. J Toxicol Environ Health B Crit Rev. 2010;13(2-4):51–138.
Chen G, Shen Y, Li X, Jiang Q, Cheng S. Gu Y, et al. The endoplasmic reticulum stress inducer thapsigargin enhances the toxicity of ZnO nanoparticles to macrophages and macrophage-endothelial co-culture. Environ Toxicol Pharmacol. 2017;50:103–10.
Chen R, Huo L, Shi X, Bai R, Zhang Z, Zhao Y, et al. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano. 2014;8(3):2562–74.
Huang CC, Aronstam RS, Chen DR, Huang YW. Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol In Vitro. 2010;24(1):45–55.
Zhao J. Xu L, Zhang T, Ren G, Yang Z. Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. Neurotoxicology. 2009;30(2):220–30.
Meshik X, Choi M, Baker A, Malchow RP, Covnot L, Doan S, et al. Modulation of voltage-gated conductances of retinal horizontal cells by UV-excited TiO2 nanoparticles. Nanomedicine. 2017;13(3):1031–40.
Huo L, Chen R, Zhao L, Shi X, Bai R, Long D, et al. Silver nanoparticles activate endoplasmic reticulum stress signaling pathway in cell and mouse models: The role in toxicity evaluation. Biomaterials. 2015;61:307–15.
Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3(11):1941–9.
Uboldi C, Bonacchi D, Lorenzi G, Hermanns MI, Pohl C, Baldi G, et al. Gold nanoparticles induce cytotoxicity in the alveolar type-II cell lines A549 and NCIH441. Part Fibre Toxicol. 2009;6:18.
Piret JP, Bondarenko OM, Boyles MSP, Himly M, Ribeiro AR, Benetti F, et al. Pan-European inter-laboratory studies on a panel of in vitro cytotoxicity and pro-inflammation assays for nanoparticles. Arch Toxicol. 2017;91(6):2315–30.
Vetten MA, Tlotleng N, Tanner Rascher D, Skepu A, Keter FK, Boodhia K, et al. Label-free in vitro toxicity and uptake assessment of citrate stabilised gold nanoparticles in three cell lines. Part Fibre Toxicol. 2013;10:50.
Austin LA, Ahmad S, Kang B, Rommel KR, Mahmoud M, Peek ME, et al. Cytotoxic effects of cytoplasmic-targeted and nuclear-targeted gold and silver nanoparticles in HSC-3 cells—a mechanistic study. Toxicol In Vitro. 2015;29(4):694–705.
Wang X, Duch MC, Mansukhani N, Ji ZX, Liao YP, Wang MY, et al. Use of a pro-fibrogenic mechanism-based predictive toxicological approach for tiered testing and decision analysis of carbonaceous nanomaterials. ACS Nano. 2015;9(3):3032–43.
Li R, Ji Z, Chang CH, Dunphy DR, Cai X, Meng H, et al. Surface interactions with compartmentalized cellular phosphates explain rare earth oxide nanoparticle hazard and provide opportunities for safer design. ACS Nano. 2014;8(2):1771–83.
Guo C, Wang J, Yang M, Li Y, Cui S, Zhou X, et al. Amorphous silica nanoparticles induce malignant transformation and tumorigenesis of human lung epithelial cells via P53 signaling. Nanotoxicology. 2017;11(9-10):1176–94.
Li Y, Liu Y. Fu Y, Wei T, Le Guyader L, Gao G, et al. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials. 2012;33(2):402–11.
Hackenberg S, Friehs G, Kessler M, Froelich K, Ginzkey C, Koehler C, et al. Nanosized titanium dioxide particles do not induce DNA damage in human peripheral blood lymphocytes. Environ Mol Mutagen. 2011;52(4):264–8.
Hackenberg S, Friehs G, Froelich K, Ginzkey C, Koehler C, Scherzed A, et al. Intracellular distribution, geno- and cytotoxic effects of nanosized titanium dioxide particles in the anatase crystal phase on human nasal mucosa cells. Toxicol Lett. 2010;195(1):9–14.
Hackenberg S, Scherzed A, Kessler M, Hummel S, Technau A, Froelich K, et al. Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett. 2011;201(1):27–33.
Hackenberg S, Scherzed A, Technau A, Kessler M, Froelich K, Ginzkey C, et al. Cytotoxic, genotoxic and pro-inflammatory effects of zinc oxide nanoparticles in human nasal mucosa cells in vitro. Toxicol In Vitro. 2011;25(3):657–63.
Hackenberg S, Zimmermann FZ, Scherzed A, Friehs G, Froelich K, Ginzkey C, et al. Repetitive exposure to zinc oxide nanoparticles induces DNA damage in human nasal mucosa mini organ cultures. Environ Mol Mutagen. 2011;52(7):582–9.
Jiang X, Foldbjerg R, Miclaus T, Wang L, Singh R, Hayashi Y, et al. Multi-platform genotoxicity analysis of silver nanoparticles in the model cell line CHO-K1. Toxicol Lett. 2013;222(1):55–63.
George JM, Magogotya M, Vetten MA, Buys AV, Gulumian M. From the cover: an investigation of the genotoxicity and interference of gold nanoparticles in commonly used in vitro mutagenicity and genotoxicity assays. Toxicol Sci. 2017;156(1):149–66.
Huk A, Izak-Nau E, El Yamani N, Uggerud H, Vadset M, Zasonska B, et al. Impact of nanosilver on various DNA lesions and HPRT gene mutations - effects of charge and surface coating. Part Fibre Toxicol. 2015;12:25.
Wang P, Wang Y, Nie X, Braïni C, Bai R, Chen C. Multiwall carbon nanotubes directly promote fibroblast–myofibroblast and epithelial–mesenchymal transitions through the activation of the TGF-β/Smad signaling pathway. Small. 2015;11(4):446–55.
Wang P, Nie X, Wang Y, Li Y, Ge C, Zhang L, et al. Multiwall carbon nanotubes mediate macrophage activation and promote pulmonary fibrosis through TGF-β/Smad signaling pathway. Small. 2013;9(22):3799–811.
Leist M, Ghallab A, Graepel R, Marchan R, Hassan R, Bennekou SH, et al. Adverse outcome pathways: opportunities, limitations and open questions. Arch Toxicol. 2017;91(11):3477–505.
Nalwa HS, Zhao YL. Nanotoxicology. Valencia: American Scientific Publishers; 2007.
Zhang L, Bai R, Liu Y, Meng L, Li B, Wang L, et al. The dose-dependent toxicological effects and potential perturbation on the neurotransmitter secretion in brain following intranasal instillation of copper nanoparticles. Nanotoxicology. 2012;6(5):562–75.
Yin H, Chen R, Casey PS, Ke PC, Davis TP, Chen C. Reducing the cytotoxicity of ZnO nanoparticles by a pre-formed protein corona in a supplemented cell culture medium. RSC Adv. 2015;5(90):73963–73.
Chen R, Zhao L, Bai R, Liu Y, Han L. Xu Z, et al. Silver nanoparticles induced oxidative and endoplasmic reticulum stresses in mouse tissues: implications for the development of acute toxicity after intravenous administration. Toxicology Research. 2016;5(2):602–8.
Wang J, Liu Y, Jiao F, Lao F, Li W. Gu Y, et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology. 2008;254(1-2):82–90.
Fahmy B, Cormier SA. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol In Vitro. 2009;23(7):1365–71.
Gabriel C, Camins A, Sureda FX, Aquirre L, Escubedo E, Pallas M, et al. Determination of nitric oxide generation in mammalian neurons using dichlorofluorescin diacetate and flow cytometry. J Pharmacol Toxicol Methods. 1997;38(2):93–8.
Dubey A, Goswami M, Yadav K, Chaudhary D. Oxidative stress and nano-toxicity induced by TiO2 and ZnO on Wag cell line. PLoS One. 2015;10(5):e0127493.
Das B, Dadhich P, Pal P, Srivas PK, Bankoti K, Dhara S. Carbon nanodots from date molasses: new nanolights for the in vitro scavenging of reactive oxygen species. J Mater Chem B. 2014;2(39):6839–47.
Aitken RJ. Nitroblue tetrazolium (NBT) assay. Reprod Biomed Online. 2018;36(1):90–1.
Chen R, Shi XF, Bai R, Rang WQ, Huo LL, Zhao L, et al. Airborne nanoparticle pollution in a wire electrical discharge machining workshop and potential health risks. Aerosol Air Qual Res. 2015;15(1):284–94.
Kang KW, Lee SJ, Kim SG. Molecular mechanism of NRF2 activation by oxidative stress. Antioxid Redox Signal. 2005;7(11-12):1664–73.
Yin H, Casey PS. Effects of iron or manganese doping of ZnO nanoparticles on their dissolution, ROS generation and cytotoxicity. RSC Adv. 2014;4(50):26149–57.
Prasad RY, McGee JK, Killius MG, Suarez DA, Blackman CF, DeMarini DM, et al. Investigating oxidative stress and inflammatory responses elicited by silver nanoparticles using high-throughput reporter genes in HepG2 cells: effect of size, surface coating, and intracellular uptake. Toxicol In Vitro. 2013;27(6):2013–21.
Le TC, Yin H, Chen R, Chen Y, Zhao L, Casey PS, et al. An experimental and computational approach to the development of ZnO nanoparticles that are safe by design. Small. 2016;12(26):3568–77.
Morry J, Ngamcherdtrakul W, Yantasee W. Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017;11:240–53.
Ahamed M, Akhtar MJ, Khan MAM, Alhadlaq HA, Aldalbahi A. Nanocubes of indium oxide induce cytotoxicity and apoptosis through oxidative stress in human lung epithelial cells. Colloids Surf B. 2017;156:157–64.
Nguyen KC, Willmore WG, Tayabali AF. Cadmium telluride quantum dots cause oxidative stress leading to extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2 cells. Toxicology. 2013;306(2):114–23.
Liu Q, Baumgartner J, Zhang Y, Liu Y, Sun Y, Zhang M. Oxidative potential and inflammatory impacts of source apportioned ambient air pollution in Beijing. Environ Sci Technol. 2014;48(21):12920–9.
Magnani ND, Muresan XM, Belmonte G, Cervellati F, Sticozzi C, Pecorelli A, et al. Skin damage mechanisms related to airborne particulate matter exposure. Toxicol Sci. 2016;149(1):227–36.
Ovrevik J, Refsnes M, Lag M, Holme JA, Schwarze PE. Activation of proinflammatory responses in cells of the airway mucosa by particulate matter: oxidant- and non-oxidant-mediated triggering mechanisms. Biomolecules. 2015;5(3):1399–440.
Chen R, Ling D, Zhao L, Wang S, Liu Y, Bai R, et al. Parallel comparative studies on mouse toxicity of oxide nanoparticle- and gadolinium-based T1 MRI contrast agents. ACS Nano. 2015;9(12):12425–35.
Foldbjerg R, Olesen P, Hougaard M, Dang DA, Hoffmann HJ, Autrup H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol Lett. 2009;190(2):156–62.
Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425.
Yuan H, Zhang Q, Guo J, Zhang T, Zhao J, Li J, et al. A PGC-1α-mediated transcriptional network maintains mitochondrial redox and bioenergetic homeostasis against doxorubicin-induced toxicity in human cardiomyocytes: implementation of TT21C. Toxicol Sci. 2016;150(2):400–17.
Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86(1):369–408.
Cole SP. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother Pharmacol. 1986;17(3):259–63.
Guo C, Wang J, Jing L, Ma R, Liu X, Gao L, et al. Mitochondrial dysfunction, perturbations of mitochondrial dynamics and biogenesis involved in endothelial injury induced by silica nanoparticles. Environ Pollut. 2018;236(2018):926–36.
Huo L, Chen R, Shi X, Bai R, Wang P, Chang Y, et al. High-content screening for assessing nanomaterial toxicity. J Nanosci Nanotechnol. 2015;15(2):1143–9.
Berridge MJ. Unlocking the secrets of cell signaling. Annu Rev Physiol. 2005;67(1):1–21.
Wang D, Guo D, Bi H. Wu Q, Tian Q, Du Y. Zinc oxide nanoparticles inhibit Ca2+-ATPase expression in human lens epithelial cells under UVB irradiation. Toxicol In Vitro. 2013;27(8):2117–26.
Guo D, Bi H, Wang D, Wu Q. Zinc oxide nanoparticles decrease the expression and activity of plasma membrane calcium ATPase, disrupt the intracellular calcium homeostasis in rat retinal ganglion cells. Int J Biochem Cell Biol. 2013;45(8):1849–59.
Zieminska E, Stafiej A, Struzynska L. The role of the glutamatergic NMDA receptor in nanosilver-evoked neurotoxicity in primary cultures of cerebellar granule cells. Toxicology. 2014;315:38–48.
Haase A, Rott S, Mantion A, Graf P, Plendl J, Thunemann AF, et al. Effects of silver nanoparticles on primary mixed neural cell cultures: uptake, oxidative stress and acute calcium responses. Toxicol Sci. 2012;126(2):457–68.
Yin N, Liu Q, Liu J, He B, Lin C, Li Z, et al. Silver nanoparticle exposure attenuates the viability of rat cerebellum granule cells through apoptosis coupled to oxidative stress. Small. 2013;9(9-10):1831–41.
Chen EY, Garnica M, Wang YC, Mintz AJ, Chen CS, Chin WCA. mixture of anatase and rutile TiO2 nanoparticles induces histamine secretion in mast cells. Part Fibre Toxicol. 2012;9:2.
Liu Y, Zhao Y, Sun B, Chen C. Understanding the toxicity of carbon nanotubes. Acc Chem Res. 2013;46(3):702–13.
Rasheed PA, Lee JS. Ultrasensitive colorimetric detection of NF-κB protein at picomolar levels using target-induced passivation of nanoparticles. Anal Bioanal Chem. 2018;410(4):1397–403.
Ge C, Meng L, Xu L, Bai R, Du J, Zhang L, et al. Acute pulmonary and moderate cardiovascular responses of spontaneously hypertensive rats after exposure to single-wall carbon nanotubes. Nanotoxicology. 2012;6(5):526–42.
Luo M, Chen P, Wang JJ, Deng XY, Dong L, Wu MH, et al. The cytotoxicity of oxidized multi-walled carbon nanotubes on macrophages. Sci China Chem. 2016;59(7):918–26.
Gordon CJ, Schladweiler MC, Krantz T, King C, Cardiovascular KUP. thermoregulatory responses of unrestrained rats exposed to filtered or unfiltered diesel exhaust. Inhal Toxicol. 2012;24(5):296–309.
Chen R, Zhang L, Ge C, Tseng MT, Bai R. Qu Y, et al. Subchronic toxicity and cardiovascular responses in spontaneously hypertensive rats after exposure to multiwalled carbon nanotubes by intratracheal instillation. Chem Res Toxicol. 2015;28(3):440–50.
Wang X, Xia T, Ntim SA, Ji Z, Lin S, Meng H, et al. Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung. ACS Nano. 2011;5(12):9772–87.
Sun B, Wang X, Ji Z, Li R, Xia T. NLRP3 inflammasome activation induced by engineered nanomaterials. Small. 2013;9(9-10):1595–607.
Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol. 2009;6(1):35.
Chen ZY, Liu Y, Sun BY, Li H, Dong JQ, Zhang LJ, et al. Polyhydroxylated metallofullerenols stimulate IL-1β secretion of macrophage through TLRs/MyD88/NF-κB pathway and NLRP3 inflammasome activation. Small. 2014;10(12):2362–72.
Liang X, Zhang D, Liu W, Yan Y, Zhou F, Wu W, et al. Reactive oxygen species trigger NF-κB-mediated NLRP3 inflammasome activation induced by zinc oxide nanoparticles in A549 cells. Toxicol Ind Health. 2017;33(10):737–45.
Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10(2):417–26.
Wang L, Luanpitpong S, Castranova V, Tse W. Lu Y, Pongrakhananon V, et al. Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett. 2011;11(7):2796–803.
Luanpitpong S, Wang L, Stueckle TA, Tse W, Chen YC, Rojanasakul Y. Caveolin-1 regulates lung cancer stem-like cell induction and p53 inactivation in carbon nanotube-driven tumorigenesis. Oncotarget. 2014;5(11):3541–54.
Zhang W, Liu HTMAPK. signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18.
Chen J, Zhang J, Cao J, Xia Z, Gan J, Inflammatory MAPK. and NF-κB signaling pathways differentiated hepatitis potential of two agglomerated titanium dioxide particles. J Hazard Mater. 2016;304:370–8.
Nie X, Tang J, Liu Y, Cai R, Miao Q, Zhao Y, et al. Fullerenol inhibits the cross-talk between bone marrow-derived mesenchymal stem cells and tumor cells by regulating MAPK signaling. Nanomedicine. 2017;13(6):1879–90.
Snyder-Talkington BN, Schwegler-Berry D, Castranova V, Qian Y, Guo NL. Multi-walled carbon nanotubes induce human microvascular endothelial cellular effects in an alveolar-capillary co-culture with small airway epithelial cells. Part Fibre Toxicol. 2013;10:35.
Simon-Vazquez R, Lozano-Fernandez T, Davila-Grana A, Gonzalez-Fernandez A. Metal oxide nanoparticles interact with immune cells and activate different cellular responses. Int J Nanomed. 2016;11:4657–68.
Kim JH, Jeong MS, Kim DY. Her S, Wie MB. Zinc oxide nanoparticles induce lipoxygenase-mediated apoptosis and necrosis in human neuroblastoma SH-SY5Y cells. Neurochem Int. 2015;90:204–14.
Vidovic S, Elder J, Medihala P, Lawrence JR, Predicala B, Zhang H, et al. ZnO nanoparticles impose a panmetabolic toxic effect along with strong necrosis, inducing activation of the envelope stress response in Salmonella enterica serovar Enteritidis. Antimicrob Agents Chemother. 2015;59(6):3317–28.
Rong LI, Wang XW, Yang XH, Gao S. Cell terminal fate—cell death. Chin Sci Bull. 2016;61(18):1983.
Jiang X, Wang L, Ji Y, Tang J, Tian X, Cao M, et al. Interference of steroidogenesis by gold nanorod core/silver shell nanostructures: implications for reproductive toxicity of silver nanomaterials. Small. 2017;13(10):1602855.
Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3(7):423–8.
Magdolenova Z, Collins A, Kumar A, Dhawan A, Stone V, Dusinska M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology. 2014;8(3):233–78.
Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85(7):743–50.
Hudecova A, Kusznierewicz B, Hasplova K, Huk A, Magdolenova Z, Miadokova E, et al. Gentiana asclepiadea exerts antioxidant activity and enhances DNA repair of hydrogen peroxide- and silver nanoparticles-induced DNA damage. Food Chem Toxicol. 2012;50(9):3352–9.
Hudecova A, Kusznierewicz B, Runden-Pran E, Magdolenova Z, Hasplova K, Rinna A, et al. Silver nanoparticles induce premutagenic DNA oxidation that can be prevented by phytochemicals from Gentiana asclepiadea. Mutagenesis. 2012;27(6):759–69.
Sanderson BJ, Johnson KJ, Henner WD. Dose-dependent cytotoxic and mutagenic effects of antineoplastic alkylating agents on human lymphoblastoid cells. Environ Mol Mutagen. 1991;17(4):238–43.
Wang JJ, Sanderson BJ, Wang H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res. 2007;628(2):99–106.
Chen Z, Wang Y, Ba T, Li Y, Pu J, Chen T, et al. Genotoxic evaluation of titanium dioxide nanoparticles in vivo and in vitro. Toxicol Lett. 2014;226(3):314–9.
Wang JJ, Wang H, Sanderson BJS. Ultrafine quartz-induced damage in human lymphoblastoid cells in vitro using three genetic damage end-points. Toxicol Mech Methods. 2007;17(4):223–32.
Hinderliter PM, Minard KR, Orr G, Chrisler WB, Thrall BD, Pounds JG, et al. ISDD: a computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part Fibre Toxicol. 2010;7(1):36.
Jiang X, Miclaus T, Wang L, Foldbjerg R, Sutherland DS, Autrup H, et al. Fast intracellular dissolution and persistent cellular uptake of silver nanoparticles in CHO-K1 cells: implication for cytotoxicity. Nanotoxicology. 2015;9(2):181–9.
Wang L, Jiang X, Ji Y, Bai R, Zhao Y. Wu X, et al. Surface chemistry of gold nanorods: origin of cell membrane damage and cytotoxicity. Nanoscale. 2013;5(18):8384–91.
DeLoid G, Cohen JM, Darrah T, Derk R, Rojanasakul L, Pyrgiotakis G, et al. Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat Commun. 2014;5(3):3514.
DeLoid GM, Cohen JM, Pyrgiotakis G, Preparation DP. characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat Protoc. 2017;12(2):355–71.
Jing X, Park JH, Peters TM, Thorne PS. Toxicity of copper oxide nanoparticles in lung epithelial cells exposed at the air-liquid interface compared with in vivo assessment. Toxicol In Vitro. 2015;29(3):502–11.
Jonathan G, Ananth T, Jones GDD, Paul H. DNA damage of macrophages induced by metal nanoparticulates using an air-liquid interface exposure model. Nanotoxicology. 2013;7(5):961–2.
Lenz AG, Karg E, Lentner B, Dittrich V, Brandenberger C, Rothen-Rutishauser B, et al. A dose-controlled system for air-liquid interface cell exposure and application to zinc oxide nanoparticles. Part Fibre Toxicol. 2009;6(1):32.
Rach J, Budde J, Mohle N, Aufderheide M. Direct exposure at the air-liquid interface: evaluation of an in vitro approach for simulating inhalation of airborne substances. J Appl Toxicol. 2014;34(5):506–15.
Shi X, Chen R, Huo L, Zhao L, Bai R, Long D, et al. Evaluation of nanoparticles emitted from printers in a clean chamber, a copy center and office rooms: health risks of indoor air quality. J Nanosci Nanotechnol. 2015;15(12):9554–64.
Chen R, Hu B, Liu Y, Xu J, Yang G, Xu D, et al. Beyond PM2.5: the role of ultrafine particles on adverse health effects of air pollution. Biochim Biophys Acta. 2016;1860(12):2844–55.
Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–7.
Service RF. Nanotechnology. Can high-speed tests sort out which nanomaterials are safe? Science. 2008;321(5892):1036–7.
Pereira DA, Williams JA. Origin and evolution of high throughput screening. Br J Pharmacol. 2007;152(1):53–61.
Zanella F, Lorens JB, Link W. High content screening: seeing is believing. Trends Biotechnol. 2010;28(5):237–45.
Donato MT, Tolosa L, Jimenez N, Castell JV, Gomez-Lechon MJ. High-content imaging technology for the evaluation of drug-induced steatosis using a multiparametric cell-based assay. J Biomol Screen. 2012;17(3):394–400.
Amorim MJB, Roca CP, Scott-Fordsmand JJ. Effect assessment of engineered nanoparticles in solid media - current insight and the way forward. Environ Pollut. 2016;218:1370–5.
Revel M, Chatel A, Mouneyrac C. Omics tools: new challenges in aquatic nanotoxicology? Aquat Toxicol. 2017;193:72–85.
Matysiak M, Kapka-Skrzypczak L, Brzoska K, Gutleb AC, Kruszewski M. Proteomic approach to nanotoxicity. J Proteomics. 2016;137:35–44.
Hartung T. Making big sense from big data in toxicology by read-across. ALTEX. 2016;33(2):83–93.
Ouedraogo M, Baudoux T, Stevigny C, Nortier J, Colet JM, Efferth T, et al. Review of current and "omics" methods for assessing the toxicity (genotoxicity, teratogenicity and nephrotoxicity) of herbal medicines and mushrooms. J Ethnopharmacol. 2012;140(3):492–512.
Kavlock RJ, Ankley GT, Blancato JN, Collette TW, Francis EZ, Gray Jr. LE, Hammerstrom K, Swartout J, Tilson HA, Toth GP, Veith GD, Weber EJ, Wolf DC, Young DM. A framework for a computational toxicology research program in ORD. U.S. Environmental Protection Agency, Washington, DC, EPA 600/R-03/065 (NTIS PB2005-105438), 2003.
Crawford SE, Hartung T, Hollert H, Mathes B, van Ravenzwaay B, Steger-Hartmann T, et al. Green toxicology: a strategy for sustainable chemical and material development. Environ Sci Eur. 2017;29(1):16.
Oki NO, Edwards SW. An integrative data mining approach to identifying adverse outcome pathway signatures. Toxicology. 2016;350-352:49–61.
Ren CX, Xiangang HU, Zhou QX. Review on attenuation of nanotoxicity and the mechanisms. Chin Sci Bull. 2016;61(7):707–17.
Tian L, Shang Y, Chen R, Bai R, Chen C, Inthavong K, et al. A combined experimental and numerical study on upper airway dosimetry of inhaled nanoparticles from an electrical discharge machine shop. Part Fibre Toxicol. 2017;14(1):24.
Bachler G, von Goetz N, Hungerbuhler K. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles. Nanotoxicology. 2015;9(3):373–80.
Li M, Panagi Z, Avgoustakis K, Reineke J. Physiologically based pharmacokinetic modeling of PLGA nanoparticles with varied mPEG content. Int J Nanomed. 2012;7:1345–56.
Bachler G, von Goetz N, Hungerbuhler K. A physiologically based pharmacokinetic model for ionic silver and silver nanoparticles. Int J Nanomed. 2013;8:3365–82.
Stearns R, Murthy G, Skornik W, Hatch V, Katler M, Godleski J. Detection of ultrafine copper oxide particles in the lungs of hamsters by electron spectroscopic imaging. In: Jouffrey B, Colliex C, editors. Proceedings of ICEM 13-PARIS. Paris: Les Editions de Physique; 1994. p.763–4.
Oberdorster G, Oberdorster E, Nanotoxicology OJ. an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005;113(7):823–39.
Semmler M, Seitz J, Erbe F, Mayer P, Heyder J, Oberdorster G, et al. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal Toxicol. 2004;16(6-7):453–9.
Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 2002;65(20):1513–30.
Cassee FR, Muijser H, Duistermaat E, Freijer JJ, Geerse KB, Marijnissen JC, et al. Particle size-dependent total mass deposition in lungs determines inhalation toxicity of cadmium chloride aerosols in rats. Application of a multiple path dosimetry model. Arch Toxicol. 2002;76(5-6):277–86.
Anjilvel S, Asgharian BA. multiple-path model of particle deposition in the rat lung. Fundam Appl Toxicol. 1995;28(1):41–50.
Riebeling C, Luch A, Gotz ME. Comparative modeling of exposure to airborne nanoparticles released by consumer spray products. Nanotoxicology. 2016;10(3):343–51.
Othman M, Latif MT, Mohamed AF. Health impact assessment from building life cycles and trace metals in coarse particulate matter in urban office environments. Ecotoxicol Environ Saf. 2017;148:293–302.
Manigrasso M, Buonanno G, Stabile L, Morawska L, Avino P. Particle doses in the pulmonary lobes of electronic and conventional cigarette users. Environ Pollut. 2015;202:24–31.
Lyu Y, Zhang K, Chai F, Cheng T, Yang Q, Zheng Z, et al. Atmospheric size-resolved trace elements in a city affected by non-ferrous metal smelting: Indications of respiratory deposition and health risk. Environ Pollut. 2017;224:559–71.
Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16(6-7):437–45.
Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114(8):1172–8.
Yegambaram M, Manivannan B, Beach TG, Halden RU. Role of environmental contaminants in the etiology of Alzheimer's disease: a review. Curr Alzheimer Res. 2015;12(2):116–46.
Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque PJ. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2017;59:133–9.
Inthavong K, Zhang K, Numerical TJ. modelling of nanoparticle deposition in the nasal cavity and the tracheobronchial airway. Comput Methods Biomech Biomed Eng. 2011;14(7):633–43.
Garcia GJ, Schroeter JD, Kimbell JS. Olfactory deposition of inhaled nanoparticles in humans. Inhal Toxicol. 2015;27(8):394–403.
Dong J, Shang Y, Inthavong K, Tu J, Chen R, Bai R, et al. From the cover: comparative numerical modeling of inhaled nanoparticle deposition in human and rat nasal cavities. Toxicol Sci. 2016;152(2):284–96.
Krug HF. Nanosafety research—are we on the right track. Angew Chem. 2014;53(46):12304–19.
Acknowledgements
We thank the Ministry of Science and Technology of China (2016YFA0201600), the National Natural Science Foundation of China (11621505, 91543206, 91643102, 21777036, 21477029), the National Science Fund for Distinguished Young Scholars (11425520), the Chinese Academy of Sciences Key Research Program for Frontier Sciences (QYZDJ-SSW-SLH022), the Key Laboratory of Biomedical Effects of Nanomaterials and Nanosafety, Chinese Academy of Sciences (no. NSKF201613), and the Chinese Academy of Sciences (XDA09040400) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Published in the topical collection Analytical Developments in Advancing Safety in Nanotechnology with guest editors Lisa Holland and Wenwan Zhong.
Rights and permissions
About this article
Cite this article
Chen, R., Qiao, J., Bai, R. et al. Intelligent testing strategy and analytical techniques for the safety assessment of nanomaterials. Anal Bioanal Chem 410, 6051–6066 (2018). https://doi.org/10.1007/s00216-018-0940-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0940-y