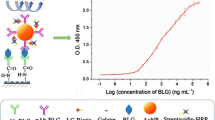
Abstract
The determination of specific IgE (sIgE) level is of great importance in IgE-mediated food allergies. Our aim was to develop a homogeneous immunoassay—light-initiated chemiluminescent assay (LICA)—for measuring allergen sIgE of a single component in egg white, thus evaluating the LICA-sIgE assay as a useful tool in the diagnosis of food allergy. The LICA-sIgE assay was performed by incubating serum sample with anti-human IgE antibody coated with chemiluminescer beads, streptavidin-coated sensitizer beads, and biotinylated antigens, which consist of four components in egg white. Serum samples from egg allergic patients (n = 70) and healthy volunteers (n = 30) were collected. For calibration, purified human IgE was used as the calibrator. Working conditions of this homogeneous immunoassay were optimized, analytical performance was determined, and correlation of the results between LICA and ImmunoCAP was evaluated. The assays were performed in 8-well plates with a sample volume diluted to 1:10 of 25 μl. Intra-assay precision (% coefficient of variation) ranged from 1.83 to 4.13%, and inter-assay precision ranged from 2.70 to 8.70%. It exhibited excellent sensitivity, which could distinguish between positive samples and negative samples even at a large dilution level. The sIgE-LICA and ImmunoCAP correlated well in patients allergic to single component (r 2 = 0.929). Also, the components ovomucoid and ovalbumin were best at predicting ImmunoCAP results, with the same area under the ROC curve (AUC) of 0.81, and a specificity of 90.0 and 93.3%, respectively. Our data show effective performance characteristics of LICA to detect sIgE in human serum based on component-resolved diagnostic tests (CRD). The homogeneous sIgE-LICA assay has the following key advantages: requires no washing, simplicity and rapidity, reproducibility, high-throughput, good performance in a liquid phase assay, and good suitability for sIgE diagnosis in food allergy based on CRD.

A light-initiated chemiluminescent assay was developed for the quantitation of sIgE against egg white allergens based on component-resolved diagnosis. Components Gal d 1 and Gal d 2 with the highest AUC values of 0.81 were considered the best at predicting egg allergy.






Similar content being viewed by others
References
Savage J, Sicherer S, Wood R. The natural history of food allergy. J Allergy Clin Immunol Pract. 2016;4(2):196–203.
Yunginger JW, Ahlstedt S, Eggleston PA, Homburger HA, Nelson HS, Ownby DR, et al. Quantitative IgE antibody assays in allergic diseases. J Allergy Clin Immunol. 2000;105(6 Pt 1):1077–84.
Dolen WK. IgE antibody in the serum-detection and diagnostic significance. Allergy. 2003;58(8):717–23.
Furuya K, Nagao M, Sato Y, Ito S, Fujisawa T. Investigators IPg. Predictive values of egg-specific IgE by two commonly used assay systems for the diagnosis of egg allergy in young children: a prospective multicenter study. Allergy. 2016;71(10):1435–43.
Johansson SG, Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology. 1967;13(4):381–94.
Lee YW, Sohn JH, Lee JH, Hong CS, Park JW. Allergen-specific IgE measurement with the IMMULITE 2000 system: intermethod comparison of detection performance for allergen-specific IgE antibodies from Korean allergic patients. Clin Chim Acta. 2009;401(1–2):25–32.
Shyur SD, Jan RL, Webster JR, Chang P, YJ L, Wang JY. Determination of multiple allergen-specific IgE by microfluidic immunoassay cartridge in clinical settings. Pediatr Allergy Immunol. 2010;21(4 Pt 1):623–33.
Park KH, Lee J, Lee SC, Son YW, Sim DW, Lee JH, et al. Comparison of the ImmunoCAP assay and AdvanSure™ AlloScreen advanced multiplex apecific IgE detection assay. Yonsei Med J. 2017;58(4):786–92.
Petersen AB, Gudmann P, Milvang-Grønager P, Mørkeberg R, Bøgestrand S, Linneberg A, et al. Performance evaluation of a specific IgE assay developed for the ADVIA centaur immunoassay system. Clin Biochem. 2004;37(10):882–92.
Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, et al. A WAO - ARIA - GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;6(1):17.
Önell A, Whiteman A, Nordlund B, Baldracchini F, Mazzoleni G, Hedlin G, et al. Allergy testing in children with persistent asthma: comparison of four diagnostic methods. Allergy. 2017;72(4):590–7.
van Erp FC, Klemans RJ, Meijer Y, van der Ent CK, Knulst AC. Using component-resolved diagnostics in the management of peanut-allergic patients. Curr Treat Options Allergy. 2016;3:169–80.
Tuano KS, Davis CM. Utility of component-resolved diagnostics in food allergy. Curr Allergy Asthma Rep. 2015;15(6):32.
Posa D, Perna S, Resch Y, Lupinek C, Panetta V, Hofmaier S, et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J Allergy Clin Immunol. 2017;139(2):541–549.e8.
Sastre J. Molecular diagnosis in allergy. Clin Exp Allergy. 2010;40(10):1442–60.
Yasgar A, Jadhav A, Simeonov A, Coussens NP. AlphaScreen-based assays: ultra-high-throughput screening for small-molecule inhibitors of challenging enzymes and protein-protein interactions. Methods Mol Biol. 2016;1439:77–98.
Asai A, Takakuma K. Alpha-based multiplexed assay for identifying SH2 domain antagonists. Methods Mol Biol. 2017;1555:351–6.
Ullman EF, Kirakossian H, Singh S, Wu ZP, Irvin BR, Pease JS, et al. Luminescent oxygen channeling immunoassay: measurement of particle binding kinetics by chemiluminescence. Proc Natl Acad Sci U S A. 1994;91(12):5426–30.
Ullman EF, Kirakossian H, Switchenko AC, Ishkanian J, Ericson M, Wartchow CA, et al. Luminescent oxygen channeling assay (LOCI): sensitive, broadly applicable homogeneous immunoassay method. Clin Chem. 1996;42(9):1518–26.
Wu F, Wang L, Guo Q, Zhao M, Gu H, Xu H, et al. A homogeneous immunoassay method for detecting interferon-gamma in patients with latent tuberculosis infection. J Microbiol Biotechnol. 2016;26(3):588–95.
Szekeres PG, Leong K, Day TA, Kingston AE, Karran EH. Development of homogeneous 384-well high-throughput screening assays for Abeta1-40 and Abeta1-42 using AlphaScreen technology. J Biomol Screen. 2008;13(2):101–11.
Kim J, Lee J, Park MR, Han Y, Shin M, Ahn K. Special consideration is required for the component-resolved diagnosis of egg allergy in infants. Ann Allergy Asthma Immunol. 2014;112(1):53–7.
Kim TE, Park SW, Cho NY, Choi SY, Yong TS, Nahm BH, et al. Quantitative measurement of serum allergen-specific IgE on protein chip. Exp Mol Med. 2002;34(2):152–8.
Bulat Lokas S, Plavec D, Rikić Pišković J, Živković J, Nogalo B, Turkalj M, et al. Allergen-specific IgE measurement: intermethod comparison of two assay systems in diagnosing clinical allergy. J Clin Lab Anal. 2017; 31(3).
Harndahl M, Justesen S, Lamberth K, Røder G, Nielsen M, Buus S. Peptide binding to HLA class I molecules: homogenous, high-throughput screening, and affinity assays. J Biomol Screen. 2009;14(2):173–80.
Wang J, Godbold JH, Sampson HA. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol. 2008;121(5):1219–24.
Villalta D, Da Re M, Conte M. Allergen component specific ige measurement with the Immulite™ 2000 system: diagnostic accuracy and intermethod comparison. J Clin Lab Anal. 2015;29(2):135–41.
Liang KL, MC S, Jiang RS. Comparison of the skin test and ImmunoCAP system in the evaluation of mold allergy. J Chin Med Assoc. 2006;69(1):3–6.
Bielefeld-Sevigny M. AlphaLISA immunoassay platform- the "no-wash" high-throughput alternative to ELISA. Assay Drug Dev Technol. 2009;7(1):90–2.
Knol EF, Knulst AC. Application of multiplexed immunoglobulin E determination on a chip in component-resolved diagnostics in allergy. Clin Exp Allergy. 2010;40(2):190–2.
Cardona V, Ansotegui IJ. Component-resolved diagnosis in anaphylaxis. Curr Opin Allergy Clin Immunol. 2016;16(3):244–9.
Ott H, Baron JM, Heise R, Ocklenburg C, Stanzel S, Merk HF, et al. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy. 2008;63(11):1521–8.
Ando H, Movérare R, Kondo Y, Tsuge I, Tanaka A, Borres MP, et al. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008;122(3):583–8.
Järvinen KM, Beyer K, Vila L, Bardina L, Mishoe M, Sampson HA. Specificity of IgE antibodies to sequential epitopes of hen's egg ovomucoid as a marker forpersistence of egg allergy. Allergy. 2007;62(7):758–65.
Gray CL, Levin ME, du Toit G. Egg sensitization, allergy and component patterns in African children with atopic dermatitis. Pediatr Allergy Immunol. 2016;27(7):709–15.
Chokshi NY, Sicherer SH. Molecular diagnosis of egg allergy: an update. Expert Rev Mol Diagn. 2015;15(7):895–906.
Acknowledgements
The authors are grateful for the technical help of Beyond, Shanghai, China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This research involved sera form allergic patients and healthy individuals. Informed consent was obtained from all human participants.
Rights and permissions
About this article
Cite this article
Bian, Y., Liu, C., She, T. et al. Development of a light-initiated chemiluminescent assay for the quantitation of sIgE against egg white allergens based on component-resolved diagnosis. Anal Bioanal Chem 410, 1501–1510 (2018). https://doi.org/10.1007/s00216-017-0791-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0791-y