Abstract
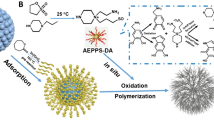
Polymeric ionic liquids (PILs) with 1-vinyl-3-ethylimidazolium cations and two different anions of Br− and PF6 − were assembled onto the surface of graphene (G) nanosheets. The derived two composites, i.e., PIL(Br)-G and PIL(PF6)-G, were further efficiently immobilized onto the surface of silica nanoparticles via self-assembly technique. The obtained two PIL-G/SiO2 nanocomposites exhibited diverse adsorption performances toward proteins through adjusting the anions of PILs. Electrostatic attractions between proteins and the nanocomposites dominated protein adsorption, while the presence of PF6 − anions weakened electrostatic interactions and deteriorated the selective adsorption of target protein, i.e., bovine serum albumin (BSA) in this case. Specifically, PIL(Br)-G/SiO2 nanocomposite displayed high selectivity toward BSA and a high adsorption efficiency of ca. 98% was achieved for 100 mg L−1 BSA in a Britton-Robinson (B-R) buffer at pH 5. HPLC analysis demonstrated the selectivity of PIL(Br)-G/SiO2 nanocomposite toward BSA in the presence of abundant hemoglobin and cytochrome c. The practical applicability was verified by performing selective isolation of human serum albumin (HSA) from human whole blood.

Selective isolation of human serum albumin from blood by polymeric ionic liquid assembled graphene immobilized silica nanocomposite with tunable anions








Similar content being viewed by others
References
Chen XW, Hu LL, Liu JW, Chen S, Wang JH. Nanoscale carbon-based materials in protein isolation and preconcentration. TRAC-Trend Anal Chem. 2013;48:30–9.
Magdeldin S, Moresco JJ, Yamamoto T, Yates JR III. Off-line multidimensional liquid chromatography and auto sampling result in sample loss in LC/LC-MS/MS. J Proteome Res. 2014;13:3826–36.
Li LP, Xu LN, Li Z, Bai Y, Liu HW. Novel nanomaterials used for sample preparation for protein analysis. Anal Bioanal Chem. 2014;406:35–47.
Bladergroen MR, van der Burgt YEM. Solid-phase extraction strategies to surmount body fluid sample complexity in high-throughput mass spectrometry-based proteomics. J Anal Methods Chem. 2015;2015:250131.
Chen LH, Zhu GS, Zhang DL, Zhao H, Guo MY, Shi W, et al. Novel mesoporous silica spheres with ultra-large pore sizes and their application in protein separation. J Mater Chem. 2009;19:2013–7.
Kryscio DR, Peppas NA. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012;8:461–73.
Li Y, Zhang XM, Deng CH. Functionalized magnetic nanoparticles for sample preparation in proteomics and peptidomics analysis. Chem Soc Rev. 2013;42:8517–39.
Chen XW, Hai X, Wang JH. Graphene/graphene oxide and their derivatives in the separation/isolation and preconcentration of protein species: a review. Anal Chim Acta. 2016;922:1–10.
Zhang YW, Li Z, Zhao Q, Zhou YL, Liu HW, Zhang XXA. Facilely synthesized amino-functionalized metal-organic framework for highly specific and efficient enrichment of glycopeptides. Chem Commun. 2014;50:11504–6.
Georgakilas V, Tiwari JN, Kemp KC, Perman JA, Bourlinos AB, Kim KS, et al. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem Rev. 2016;116:5464–519.
Zhu YW, Murali S, Cai WW, Li XS, Suk JW, Potts JR, et al. Graphene and graphene oxide: synthesis, properties and applications. Adv Mater. 2010;22:3906–24.
Yuan JY, Mecerreyes D, Antonietti M. Poly(ionic liquid)s: an update. Prog Polym Sci. 2013;38:1009–36.
Tokuda M, Yamane M, Thickett SC, Minami H, Zetterlund PB. Synthesis of polymeric nanoparticles containing reduced graphene oxide nanosheets stabilized by poly(ionic liquid) using miniemulsion polymerization. Soft Matter. 2016;12:3955–62.
Xu CX, Li Y, Liang GZ, Gu AJ. Building a poly(epoxy propylimidazolium ionic liquid)/graphene hybrid through πcation-π interaction for fabricating high-k polymer composites with low dielectric loss and percolation threshold. J Mater Chem C. 2016;4:3175–84.
Wu SY, Wang YX, Mao H, Wang C, Xia LX, Zhang Y, et al. Direct electrochemistry of cholesterol oxidase and biosensing of cholesterol on PSS/polymeric ionic liquid-graphene nanocomposite. RSC Adv. 2016;6:59487–96.
Sun M, Bu YN, Feng JJ, Luo CN. Graphene oxide reinforced polymeric ionic liquid monolith solid-phase microextraction sorbent for high-performance liquid chromatography analysis of phenolic compounds in aqueous environmental samples. J Sep Sci. 2016;39:375–82.
Zhang BT, Zheng XX, Li HF, Lin JM. Application of carbon-based nanomaterials in sample preparation: a review. Anal Chim Acta. 2013;784:1–17.
Liu CC, Deng QL, Fang GZ, Huang X, Wang S. Facile synthesis of graphene doped poly(ionic liquid) boronate affinity material for specific capture of glycoproteins. J Mater Chem B. 2014;2:5229–37.
Liu JW, Wang MM, Zhang Y, Han L, Chen XW, Wang JH. Polymeric ionic liquid modified reduced graphene oxide as adsorbent for highly selective isolation of acidic protein. RSC Adv. 2014;4:61936–43.
Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin—more than just a serum protein. Front Physiol. 2014;5:299.
Arroyo V, Garcia-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407.
Pernemalm M, Lehtio J. Mass spectrometry-based plasma proteomics: state of the art and future outlook. Expert Rev Proteomic. 2014;11:431–48.
Liu JW, Yang T, Ma LY, Chen XW, Wang JH. Nickel nanoparticle decorated graphene for highly selective isolation of polyhistidine-tagged proteins. Nanotechnology. 2013;24:505704.
Marcilla R, Blazquez JA, Rodriguez J, Pomposo JA, Mecerreyes D. Tuning the solubility of polymerized ionic liquids by simple anion-exchange reactions. J Polym Sci Polym Chem. 2004;42:208–12.
Lin ZA, Xia ZW, Zheng JN, Zheng D, Zhang L, Yang HH, et al. Synthesis of uniformly sized molecularly imprinted polymer-coated silica nanoparticles for selective recognition and enrichment of lysozyme. J Mater Chem. 2012;22:17914–22.
Kruger NJ. The protein protocols handbook. New York: Springer Humana Press; 2009. p. 17–24.
Kim TY, Lee HW, Stoller M, Dreyer DR, Bielawski CW, Ruoff RS, et al. High-performance supercapacitors based on poly(ionic liquid)-modified graphene electrodes. ACS Nano. 2011;5:436–42.
Liu JW, Yang T, Chen S, Chen XW, Wang JH. Nickel chelating functionalization of graphene composite for metal affinity membrane isolation of lysozyme. J Mater Chem B. 2013;1:810–8.
Chen JL, Yan XP. A dehydration and stabilizer-free approach to production of stable water dispersions of graphene nanosheets. J Mater Chem. 2010;20:4328–32.
Stankovich S, Dikin DA, Piner RD, Kohlhass KA, Kleinhammes A, Jia YY, et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon. 2007;45:1558–65.
Villar-Garcia IJ, Smith EF, Taylor AW, Qiu FL, Lovelock KRJ, Jones RG, et al. Charging of ionic liquid surface under X-ray irradiation: the measurement of absolute binding energies by XPS. Phys Chem Chem Phys. 2011;13:2797–808.
Liu N, Luo F, Wu HX, Liu YH, Zhang C, Chen J. One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv Funct Mater. 2008;18:1518–25.
Jones KL, O’Melia CR. Protein and humic acid adsorption onto hydrophilic membrane surfaces: effect of pH and ionic strength. J Membrane Sci. 2000;165:31–46.
Song H, Yang C, Yohannes A, Yao S. Acidic ionic liquid modified silica gel for adsorption and separation of bovine serum albumin (BSA). RSC Adv. 2016;6:107452–62.
Liu CC, Deng QL, Fang GZ, Liu HL, Wu JH, Pan MF, et al. Ionic liquids monolithic columns for protein separation in capillary electrochromatography. Anal Chim Acta. 2013;804:313–20.
Zhu LY, Li GQ, Zheng FY. Interaction of bovine serum albumin with two alkylimidazolium-based ionic liquids investigated by microcalorimetry and circular dichroism. J Biophys Chem. 2011;2:146–51.
Liu JW, Zhang Q, Chen XW, Wang JH. Surface assembly of graphene oxide nanosheets on SiO2 particles for the selective isolation of hemoglobin. Chem Eur J. 2011;17:4864–70.
Vilar VJP, Botelho CMS, Boaventura RAR. Influence of pH, ionic strength and temperature on lead biosorption by Gelidium and agar extraction algal waste. Process Biochem. 2005;40:3267–75.
Liu JW, Yu H, Liang QM, Liu YN, Shen JW, Bai Q. Preparation of polyhedral oligomeric silsesquioxane based cross-linked inorganic-organic nanohybrid as adsorbent for selective removal of acidic dyes from aqueous solution. J Colloid Interf Sci. 2017;497:402–12.
Zhang CJ, Jia XP, Wang YZ, Zhang M, Yang S, Guo JX. Thermosensitive molecularly imprinted hydrogel cross-linked with N-malely chitosan for the recognition and separation of BSA. J Sep Sci. 2014;37:419–26.
Zhang P, Fang XN, Yan GQ, Gao MX, Zhang XM. Highly efficient enrichment of low-abundance proteins by core-shell structured Fe3O4-chitosan@graphene composites. Talanta. 2017;174:845–52.
Zhang T, Mei ZY, Zhou YM, Yu S, Chen ZJ, Bu XH. Novel paper-templated fabrication of hierarchically porous Ni-Al layered double hydroxides/Al2O3 for efficient BSA separation. J Chem Technol Biotechnol. 2014;89:1705–11.
Mahdavinia GR, Mousanezhad S, Hosseinzadeh H, Darvishi F, Sabzi M. Magnetic hydrogel beads based on PVA/sodium alginate/laponite RD and studying their BSA adsorption. Carbohyd Polym. 2016;147:379–91.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Nos. 21605122, 21505104, 21545007, and 21575114) and the Foundation of Key Laboratory in Shaanxi Province (Nos. 16JS116 and 15JS115).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study has been approved by the ethics committee of Northwest University and has been performed in accordance with the ethical standards. Written informed consent has been obtained from the participant who provided the blood sample.
Conflict of interest
There are no conflicts to declare.
Electronic supplementary material
ESM 1
(PDF 364 KB)
Rights and permissions
About this article
Cite this article
Liu, J., Liang, Y., Shen, J. et al. Polymeric ionic liquid-assembled graphene-immobilized silica composite for selective isolation of human serum albumin from human whole blood. Anal Bioanal Chem 410, 573–584 (2018). https://doi.org/10.1007/s00216-017-0758-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0758-z