Abstract
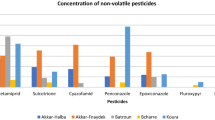
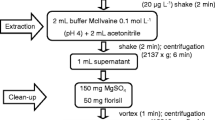
An optimized analytical method was developed for the simultaneous analysis of 90 pesticides, 16 polycyclic aromatic hydrocarbons, and 22 polychlorinated biphenyls. The method was based on quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction using acetonitrile followed by a dispersive solid-phase extraction cleanup using primary–secondary amine and octadecyl (C18). The extract obtained was concentrated by evaporation and then reconstituted with acetonitrile to prepare it for chromatographic analysis by liquid chromatography–triple-quadrupole tandem mass spectrometry and gas chromatography–ion-trap tandem mass spectrometry, which was preceded by a preconcentration step using solid-phase microextraction with appropriate fibers. The combination of the two extraction steps ensured efficient extract cleanup. The use of the two analytical instruments allowed the analysis of a large number of pollutants with a high reliability rate. The method developed was validated for linearity, which was studied with use of matrix-matched calibration curves in the concentration range between 10 and 3000 ng g−1. The correlation coefficient (R 2) obtained was higher than 0.98 for most of the target compounds, with a relative standard deviation lower than 20% for repeatability and reproducibility. The limits of detection and quantification were lower than 20 and 60 ng g-1 respectively for the compounds analyzed, and the recoveries were between 60% and 103% for most compounds. Finally, the method was tested for its efficiency on real samples by the analysis of three honey samples in which seven pesticides and nine polycyclic aromatic hydrocarbons were determined.

ᅟ
Similar content being viewed by others

References
Panseri S, Catalano A, Giorgi A, Arioli F, Procopio A, Britti D, et al. Occurrence of pesticide residues in Italian honey from different areas in relation to its potential contamination sources. Food Control. 2014;38:150–6.
Panseri S, Manzo A, Chiesa LM, Giorgi A. Melissopalynological and volatile compounds analysis of buckwheat honey from different geographical origins and their role in botanical determination. J Chem. 2013;2013:904202.
Dobrinas S, Birghila S, Coatu V. Assessment of polycyclic aromatic hydrocarbons in honey and propolis produced from various flowering trees and plants in Romania. J Food Comp Anal. 2008;21(1):71–7. doi:10.1016/j.jfca.2007.07.003.
Raeymaekers B. A prospective biomonitoring campaign with honey bees in a district of Upper-Bavaria (Germany). Environ Monit Assess. 2006;116(1-3):233–43.
Kujawski MW, Namieśnik J. Challenges in preparing honey samples for chromatographic determination of contaminants and trace residues. Trends Anal Chem. 2008;27(9):785–93. doi:10.1016/j.trac.2008.07.004.
Herrera López S, Lozano A, Sosa A, Hernando MD, Fernández-Alba AR. Screening of pesticide residues in honeybee wax comb by LC-ESI-MS/MS. A pilot study. Chemosphere. 2016;163:44–53. doi:10.1016/j.chemosphere.2016.07.008.
Machado I, Gérez N, Pistón M, Heinzen H, Cesio MV. Determination of pesticide residues in globe artichoke leaves and fruits by GC–MS and LC–MS/MS using the same QuEChERS procedure. Food Chem. 2017;227:227–36. doi:10.1016/j.foodchem.2017.01.025.
Karami-Mohajeri S, Abdollahi M. Toxic effects of organophosphate, carbamate, and organochlorine pesticides on cellular metabolism of lipids, proteins, and carbohydrates: a comprehensive review. Hum Exp Toxicol. 2011;30(9):1119–40.
Zheng S, Chen B, Qiu X, Chen M, Ma Z, Yu X. Distribution and risk assessment of 82 pesticides in Jiulong River and estuary in South China. Chemosphere. 2016;144:1177–92.
Zhou R, Zhu L, Chen Y, Kong Q. Concentrations and characteristics of organochlorine pesticides in aquatic biota from Qiantang River in China. Environ Pollut. 2008;151(1):190–9.
de Lima RF, Dionello RG, Peralba MCR, Barrionuevo S, Radunz LL. Reichert Júnior FW. PAHs in corn grains submitted to drying with firewood. Food Chem. 2017;215:165–70. doi:10.1016/j.foodchem.2016.07.164.
Fiandanese N, Borromeo V, Berrini A, Fischer B, Schaedlich K, Schmidt J-S, et al. Maternal exposure to a mixture of di(2-ethylhexyl) phthalate (DEHP) and polychlorinated biphenyls (PCBs) causes reproductive dysfunction in adult male mouse offspring. Reprod Toxicol. 2016;65:123–32. doi:10.1016/j.reprotox.2016.07.004.
Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Petroleum. 2016;25(1):107–23.
Elorduy I, Elcoroaristizabal S, Durana N, García J, Alonso L. Diurnal variation of particle-bound PAHs in an urban area of Spain using TD-GC/MS: influence of meteorological parameters and emission sources. Atmos Environ. 2016;138:87–98.
Kodavanti PRS. Polychlorinated biphenyls (PCBs)☆. In: Reference module in neuroscience and biobehavioral psychology. Elsevier; 2017. doi:10.1016/B978-0-12-809324-5.03955-9.
Amendola G, Pelosi P, Dommarco R. Solid-phase extraction for multi-residue analysis of pesticides in honey. J Environ Sci Health B. 2010;46(1):24–34.
Kujawski MW, Namieśnik J. Levels of 13 multi-class pesticide residues in Polish honeys determined by LC-ESI-MS/MS. Food Control. 2011;22(6):914–9.
Rissato SR, Galhiane MS, Knoll FR, Apon BM. Supercritical fluid extraction for pesticide multiresidue analysis in honey: determination by gas chromatography with electron-capture and mass spectrometry detection. J Chromatogr A. 2004;1048(2):153–9.
Sanchez-Brunete C, Albero B, Miguel E, Tadeo JL. Determination of insecticides in honey by matrix solid-phase dispersion and gas chromatography with nitrogen–phosphorus detection and mass spectrometric confirmation. J AOAC Int. 2002;85(1):128–33.
Chiesa LM, Labella GF, Giorgi A, Panseri S, Pavlovic R, Bonacci S, et al. The occurrence of pesticides and persistent organic pollutants in Italian organic honeys from different productive areas in relation to potential environmental pollution. Chemosphere. 2016;154:482–90. doi:10.1016/j.chemosphere.2016.04.004.
Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int. 2003;86(2):412–31.
Albinet A, Tomaz S, Lestremau F. A really quick easy cheap effective rugged and safe (QuEChERS) extraction procedure for the analysis of particle-bound PAHs in ambient air and emission samples. Sci Total Environ. 2013;450:31–8.
Wilkowska A, Biziuk M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011;125(3):803–12.
Arthur CL, Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem. 1990;62(19):2145–8.
Li J, Wang Y-B, Li K-Y, Cao Y-Q, Wu S, Wu L. Advances in different configurations of solid-phase microextraction and their applications in food and environmental analysis. Trends Anal Chem. 2015;72:141–52. doi:10.1016/j.trac.2015.04.023.
Bojko B, Pawliszyn J. In vivo and ex vivo SPME: a low invasive sampling and sample preparation tool in clinical bioanalysis. Bioanalysis. 2014;6(9):1227–39.
Megson D, Reiner EJ, Jobst KJ, Dorman FL, Robson M, Focant J-F. A review of the determination of persistent organic pollutants for environmental forensics investigations. Anal Chim Acta. 2016. doi:10.1016/j.aca.2016.08.027.
García-Valcárcel AI, Molero E, Tadeo JL, Hernando MD. Determination of selected environmental contaminants in foraging honeybees. Talanta. 2016;148:1–6. doi:10.1016/j.talanta.2015.10.064.
Díez C, Traag WA, Zommer P, Marinero P, Atienza J. Comparison of an acetonitrile extraction/partitioning and “dispersive solid-phase extraction” method with classical multi-residue methods for the extraction of herbicide residues in barley samples. J Chromatogr A. 2006;1131(1–2):11–23. doi:10.1016/j.chroma.2006.07.046.
Wiest L, Buleté A, Giroud B, Fratta C, Amic S, Lambert O, et al. Multi-residue analysis of 80 environmental contaminants in honeys, honeybees and pollens by one extraction procedure followed by liquid and gas chromatography coupled with mass spectrometric detection. J Chromatogr A. 2011;1218(34):5743–56.
Ouyang G, Jiang R (eds) (2016) Solid phase microextraction: recent developments and applications. Springer, Berlin
Walorczyk S, Gnusowski B. Development and validation of a multi-residue method for the determination of pesticides in honeybees using acetonitrile-based extraction and gas chromatography–tandem quadrupole mass spectrometry. J Chromatogr A. 2009;1216(37):6522–31.
Shabir GA. Validation of high-performance liquid chromatography methods for pharmaceutical analysis: Understanding the differences and similarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. J Chromatogr A. 2003;987(1–2):57–66. doi:10.1016/S0021-9673(02)01536-4.
Malhat FM, Haggag MN, Loutfy NM, Osman MA, Ahmed MT. Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemosphere. 2015;120:457–61.
Moniruzzaman M, Rodríguez I, Ramil M, Cela R, Sulaiman SA, Gan SH. Assessment of gas chromatography time-of-flight accurate mass spectrometry for identification of volatile and semi-volatile compounds in honey. Talanta. 2014;129:505–15. doi:10.1016/j.talanta.2014.06.019.
Acknowledgements
We gratefully acknowledge AZM & SAADE Association and the Lebanese University for funding the project, as well as Strasbourg University for international mobility aid, without which the present study could not have been completed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Al-Alam, J., Fajloun, Z., Chbani, A. et al. A multiresidue method for the analysis of 90 pesticides, 16 PAHs, and 22 PCBs in honey using QuEChERS–SPME. Anal Bioanal Chem 409, 5157–5169 (2017). https://doi.org/10.1007/s00216-017-0463-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0463-y