Abstract
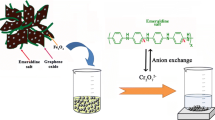
A fast, simple, economical, and environmentally friendly magnetic solid-phase extraction (MSPE) procedure has been developed to preconcentrate 2,4,6-trinitrotoluene (TNT) from water samples prior to determination by liquid chromatography-UV-Vis employing graphene oxide/Fe3O4 nanocomposite as sorbent. The nanocomposite synthesis was investigated, and the MSPE was optimized by a multivariate approach. The optimum MSPE conditions were 40 mg of nanocomposite, 10 min of vortex extraction, 1 mL of acetonitrile as eluent, and 6 min of desorption in an ultrasonic bath. Under the optimized experimental conditions, the method was evaluated to obtain a preconcentration factor of 153. The linearity of the method was studied from 1 to 100 μg L−1 (N = 5), obtaining a correlation coefficient of 0.994. The relative standard deviation and limit of detection were found to be 12% (n = 6, 10 μg L−1) and 0.3 μg L−1, respectively. The applicability of the method was investigated, analyzing three types of water samples (i.e., reservoir and drinking water and effluent wastewater) and recovery values ranged between 87 and 120% (50 μg L−1 spiking level), showing that the matrix had a negligible effect upon extraction. Finally, the semiquantitative Eco-Scale metrics confirmed the greenness of the developed method.






Similar content being viewed by others
References
Stoller MD, Park S, Zhu Y, An J, Ruoff RS. Graphene-based ultracapacitors. Nano Lett. 2008;8:3498–502.
Lee C, Wei X, Kysar JW, Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321:385–88.
Bolotin KI, Sikes KJ, Jiang Z, Klima M, Fudenberg G, Hone J, et al. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008;146:351–55.
Liu Q, Shi J, Zeng L, Wang T, Cai Y, Jiang G. Evaluation of graphene as an advantageous adsorbent for solid-phase extraction with chlorophenols as model analytes. J Chromatogr A. 2011;1218:197–204.
Chen J, Zou J, Zeng J, Song X, Ji J, Wang Y, et al. Preparation and evaluation of graphene-coated solid-phase microextraction fiber. Anal Chim Acta. 2010;678:44–9.
Zhang H, Lee HK. Plunger-in-needle solid-phase microextraction with graphene-based sol–gel coating as sorbent for determination of polybrominated diphenyl ethers. J Chromatogr A. 2011;1218:4509–16.
Zhang S, Du Z, Li G. Layer-by-layer fabrication of chemical-bonded graphene coating for solid-phase microextraction. Anal Chem. 2011;83:7531–41.
Ponnusamy VK, Jen JF. A novel graphene nanosheets coated stainless steel fiber for microwave assisted headspace solid phase microextraction of organochlorine pesticides in aqueous samples followed by gas chromatography with electron capture detection. J Chromatogr A. 2011;1218:6861–68.
Hummers WS, Offeman RE. Preparation of graphitic oxide. J Am Chem Soc. 1958;80:1339–39.
Fritz JS. Analytical solid-phase extraction. New York: Wiley; 1999.
Han Q, Wang Z, Xia J, Xia L, Chen S, Zhang XQ, et al. Graphene as an efficient sorbent for the SPE of organochlorine pesticides in water samples coupled with GC-MS. J Sep Sci. 2013;36:3586–91.
Wang Z, Han Q, Xia J, Xia L, Ding M, Tang J. Graphene-based solid-phase extraction disk for fast separation and preconcentration of trace polycyclic aromatic hydrocarbons from environmental water samples. J Sep Sci. 2013;36:1834–42.
Wu J, Chen L, Mao P, Lu Y, Wang HZ. Determination of chloramphenicol in aquatic products by graphene-based SPE coupled with HPLC-MS/MS. J Sep Sci. 2012;35:3586–92.
Luo X, Zhang FF, Ji S, Yang B, Liang X. Graphene nanoplatelets as a highly efficient solid-phase extraction sorbent for determination of phthalate esters in aqueous solution. Talanta. 2014;120:71–5.
Wang SL, Hu S, Xu H. Analysis of aldehydes in human exhaled breath condensates by in-tube SPME-HPLC. Anal Chim Acta. 2015;900:67–75.
Pawliszyn J. Solid Phase Microextraction. Theory and Practice. New York: Wiley; 1997.
He H, Klinowski J, Forster M, Lerf A. A new structural model for graphite oxide. Chem Phys Lett. 1998;287:53–6.
Sitko R, Zawisza B, Malicka E. Graphene as a new sorbent in analytical chemistry. Trends Anal Chem. 2013;51:33–43.
Yang X, Li J, Wen T, Ren X, Huang Y, Wang X. Adsorption of naphthalene and its derivatives on magnetic graphene composites and the mechanism investigation. Colloid Surface A. 2013;422:118–25.
Sitko R, Turek E, Zawisza B, Malicka E, Talik E, Heimann J, et al. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013;42:5682–89.
Liu Q, Shi J, Sun J, Wang T, Zeng L, Jiang G. Graphene and graphene oxide sheets supported on silica as versatile and high-performance adsorbents for solid-phase extraction. Angew Chem Int Ed. 2011;50:5913–17.
Xu L, Feng J, Li J, Liu X, Jiang S. Graphene oxide bonded fused-silica fiber for solid-phase microextraction-gas chromatography of polycyclic aromatic hydrocarbons in water. J Sep Sci. 2012;35:93–100.
Meng J, Shi C, Wei B, Yu W, Deng C, Zhang X. Preparation of Fe3O4@C@PANI magnetic microspheres for the extraction and analysis of phenolic compounds in water samples by gas chromatography-mass spectrometry. J Chromatogr A. 2011;1218:2841–47.
Sasaki T, Tanaka S. Adsorption behavior of some aromatic compounds on hydrophobic magnetite for magnetic separation. J Hazard Mater. 2011;196:327–34.
Zhang X, Niu H, Pan Y, Shi Y, Cai Y. Modifying the surface of Fe3O4/SiO2 magnetic nanoparticles with C18/NH2 mixed group to get an efficient sorbent for anionic organic pollutants. J Colloid Interface Sci. 2011;362:107–12.
Tang H, Zhu L, Yu C, Shen X. Selective photocatalysis mediated by magnetic molecularly imprinted polymers. Sep Purif Technol. 2012;95:165–71.
Li S, Gong Y, Yang Y, He C, Hu L, Zhu L, et al. Recyclable CNTs/Fe3O4 magnetic nanocomposites as adsorbents to remove bisphenol A from water and their regeneration. Chem Eng J. 2015;260:231–39.
Sun T, Yang J, Li L, Wang X, Li X, Jin Y. Preparation of graphene sheets with covalently bonded Fe3O4 for magnetic solid-phase extraction applied to organochlorine pesticides in orange juice. Chromatographia. 2016;79:345–53.
Han Q, Wang Z, Xia J, Chen S, Zhang X, Ding M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta. 2012;101:388–95.
Aguilar-Arteaga K, Rodriguez JA, Miranda JM, Medina J, Barrado E. Determination of non-steroidal anti-inflammatory drugs in wastewaters by magnetic matrix solid phase dispersion–HPLC. Talanta. 2010;80:1152–57.
Šafařı́k I, Šafařı́ková M. Detection of low concentrations of malachite green and crystal violet in water. Water Res. 2002;36:196-200
Taghvimi A, Hamishehkar H, Ebrahimi M. Magnetic nano graphene oxide as solid phase extraction adsorbent coupled with liquid chromatography to determine pseudoephedrine in urine samples. J Chromatogr B. 2016;1009-1010:66–72.
Zeng S, Gan N, Weideman-Mera R, Cao Y, Li T, Sang W. Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide. Chem Eng J. 2013;218:108–15.
Stucki H. Toxicity and degradation of explosives. Chimia. 2004;58:409–13.
Keith L, Telliard W. Priority pollutants I—a perspective view. Environ Sci Technol. 1979;13:416–23.
Talmage SS, Opresko DM, Maxwell CJ, Welsh CJE, Cretella FM, Reno PH, et al. Reviews of Environmental Contamination and Toxicology. New York: Springer; 1999.
Psillakis E, Kalogerakis N. Solid-phase microextraction versus single-drop microextraction for the analysis of nitroaromatic explosives in water samples. J Chromatogr A. 2011;938:113–20.
Psillakis E, Kalogerakis N. Application of solvent microextraction to the analysis of nitroaromatic explosives in water samples. J Chromatogr A. 2001;907:211–19.
Psillakis E, Mantzavinos D, Kalogerakis N. Development of a hollow fibre liquid phase microextraction method to monitor the sonochemical degradation of explosives in water. Anal Chim Acta. 2004;501:3–10.
Cortada C, Vidal L, Canals A. Determination of nitroaromatic explosives in water samples by direct ultrasound-assisted dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry. Talanta. 2011;85:2546–52.
Wei Y, Han B, Hu X, Lin Y, Wang X, Deng X. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012;27:632–7.
Shih CJ, Lin S, Sharma R, Strano MS, Blankschtein D. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: a comparative experimental and molecular dynamics simulation study. J Am Chem Soc. 2012;28:235–41.
Thermo Scientific database (November, 2016), www.lasurface.com
Draper NR. Plackett and Burman designs. In: Kotz S, Johnson L, editors. Encyclopedia of Statistical Sciences. New York: John Wiley & Sons; 1985. p. 754–8.
Gałuszka A, Konieczka P, Migaszewski ZM, Namiesnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures. Trends Anal Chem. 2012;37:61–72.
Acknowledgements
The authors would like to thank the Ministry of Science and Innovation of Spain (project no. CTQ2011-23968) for the financial support and L. Costa thanks the Capes Foundation within the Ministry of Education in Brazil (Process 12013/13-7).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 260 kb)
Rights and permissions
About this article
Cite this article
Costa dos Reis, L., Vidal, L. & Canals, A. Graphene oxide/Fe3O4 as sorbent for magnetic solid-phase extraction coupled with liquid chromatography to determine 2,4,6-trinitrotoluene in water samples. Anal Bioanal Chem 409, 2665–2674 (2017). https://doi.org/10.1007/s00216-017-0211-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0211-3