Abstract
The illegal use of pharmacologically active compounds for growth promotion in food-producing species poses risks for consumer health and animal welfare. Surveillance relies on the quantification of drug residues in animal fluids or tissues, but the efficacy can be negatively affected due to undetectable residual concentrations in biological matrices. Consequently, techniques focusing on the indirect biological effects of exogenous compound administration have been proposed as more sensitive detection methods. The purpose of the present study is to develop a tandem mass spectrometry analytical method based on low-energy collision-induced dissociation (CID-MS/MS) using multiple reaction monitoring (MRM) for the quantification of 12 potential protein markers of skeletal muscle to detect anabolic treatments with dexamethasone. Protein markers identified in a previous study applying a 2D-DIGE proteomics approach have been quantified using the signature peptide method. A group of proteins were confirmed as reliable markers. Quantitative results enabled a predictive model to be defined based on logistic regression for the detection of treated animals. The developed model was finally cross-validated in an independent animal set.

Analytical workflow used for the quantification of indirect protein markers of dexamethasone treatment






Similar content being viewed by others
References
Council Directive 96/22/EC of 29 April 1996 concerning the prohibition on the use in stockfarming of certain substances having a hormonal or thyrostatic action and of beta-agonists, and repealing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC. Off J Eur Comm. 1996;L125:3–9.
Serratosa J, Blass A, Rigau B, Mongrell B, Rigau T, Tortadès M, et al. Residues from veterinary medicinal products, growth promoters and performance enhancers in food-producing animals: a European Union perspective. Rev Sci Tech. 2006;25(2):637–53.
Rubies A, Cabrera A, Centrich F. Determination of synthetic hormones in animal urine by high-performance liquid chromatography/mass spectrometry. J AOAC Int. 2007;90(2):626–32.
De Brabander HF, Le Bizec B, Pinel G, Antignac JP, Verheyden K, Mortier V, et al. Past, present and future of mass spectrometry in the analysis of residues of banned substances in meat-producing animals. J Mass Spectrom. 2007;42(8):983–98.
Kinsella B, O’Mahony J, Malone E, Moloney M, Cantwell H, Furey A, et al. Current trends in sample preparation for growth promoter and veterinary drug residue analysis. J Chromatogr A. 2009;1216(46):7977–8015.
Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Commun. 2002;L221:8–36.
Stolker AAM, Zuidema T, Nielen MWF. Residue analysis of veterinary drugs and growth-promoting agents. Trends Anal Chem. 2007;26(10):967–79.
Nielen MW, Lasaroms JJ, Essers ML, Sanders MB, Heskamp HH, Bovee TF, et al. The ultimate veal calf reference experiment: hormone residue analysis data obtained by gas and liquid chromatography tandem mass spectrometry. Anal Chim Acta. 2007;586(1-2):30–4.
McGrath TF, van Meeuwen JA, Massart AC, de Pauw E, Delahaut P, Buijs J, et al. Effect-based proteomic detection of growth promoter abuse. Anal Bioanal Chem. 2013;405(4):1171–9.
Courtheyn D, Le Bizec B, Brambilla G, De Brabander HF, Cobbaert E, Van de Wiele M, et al. Recent developments in the use and abuse of growth promoters. Anal Chim Acta. 2002;473(1-2):71–82.
Smits NGE, Bremer MGEG, Ludwig SKJ, Nielen MWF. Development of a flow cytometric immunoassay for recombinant bovine somatotropin-induced antibodies in serum of dairy cows. Drug Test Anal. 2012;4(5):362–7.
Mooney MH, Le Bizec B, Elliott CT. Combining biomarker screening and mass-spectrometric analysis to detect hormone abuse in cattle. Trends Anal Chem. 2009;28(6):665–75.
Pinel G, Weigel S, Antignac JP, Mooney MH, Elliott C, Nielen MWF, et al. Targeted and untargeted profiling of biological fluids to screen for anabolic practices in cattle. Trends Anal Chem. 2010;29(11):1269–80.
Bozzetta E, Pezzolato M, Maurella C, Varello K, Richelmi GB, Draisci R, et al. Development of an enhanced histopathological approach to detect low-dose dexamethasone illicit treatment in veal calves. Food Addit Contam Part A: Chem Anal Control Expo Risk Assess. 2011;28(9):1187–92.
Doué M, Dervilly-Pinel G, Cesbron N, Stefani A, Moro L, Biancotto G, et al. Clinical biochemical and hormonal profiling in plasma: a promising strategy to predict growth hormone abuse in cattle. Anal Bioanal Chem. 2015;07(15):4343–9.
Ludwig SK, Smits NG, Cannizzo FT, Nielen MW. Potential of treatment-specific protein biomarker profiles for detection of hormone abuse in cattle. J Agric Food Chem. 2013;61(19):4514–9.
Courant F, Pinel G, Bichon E, Monteau F, Antignac JP, Le Bizec B. Development of a metabolomic approach based on liquid chromatography-high resolution mass spectrometry to screen for clenbuterol abuse in calves. Analyst. 2009;134(8):1637–46.
Regal P, Anizan S, Antignac JP, Le Bizec B, Cepeda A, Fente C. Metabolomic approach based on liquid chromatography coupled to high resolution mass spectrometry to screen for the illegal use of estradiol and progesterone in cattle. Anal Chim Acta. 2011;700(1-2):16–25.
Lopparelli RM, Giantin M, Pozza G, Stefani AL, Ravarotto L, Montesissa C, et al. Target gene expression signatures in neutrophils and lymphocytes from cattle administered with dexamethasone at growth promoting purposes. Res Vet Sci. 2012;93(1):226–33.
van den Broek I, Blokland M, Nessen MA, Sterk S. Current trends in mass spectrometry of peptides and proteins: application to veterinary and sports-doping control. Mass Spectrom Rev. 2015;34(6):571–94.
Pegolo S, Cannizzo FT, Biolatti B, Castagnaro M, Bargelloni L. Transcriptomic profiling as a screening tool to detect trenbolone treatment in beef cattle. Res Vet Sci. 2014;96(3):472–81.
Mooney MH, Situ C, Cacciatore G, Hutchinson T, Elliott C, Bergwerff AA. Plasma biomarker profiling in the detection of growth promoter use in calves. Biomarkers. 2008;13(3):246–56.
Draisci R, Montesissa C, Santamaria B, D’Ambrosio C, Ferretti G, Merlanti R, et al. Integrated analytical approach in veal calves administered the anabolic androgenic steroids boldenone and boldione: urine and plasma kinetic profile and changes in plasma protein expression. Proteomics. 2007;7(17):3184–93.
Della Donna L, Ronci M, Sacchetta P, Di Ilio C, Biolatti B, Federici G, et al. A food safety control low mass-range proteomics platform for the detection of illicit treatments in veal calves by MALDI-TOF-MS serum profiling. Biotechnol J. 2009;4(11):1596–609.
Guglielmetti C, Mazza M, Pagano M, Carrella S, Sciuto S, Nodari S, et al. Identification by a proteomic approach of a plasma protein as a possible biomarker of illicit dexamethasone treatment in veal calves. Food Addit Contam Part A: Chem Anal Control Expo Risk Assess. 2014;31(5):833–8.
Stella R, Biancotto G, Arrigoni G, Barrucci F, Angeletti R, James P. Proteomics for the detection of indirect markers of steroids treatment in bovine muscle. Proteomics. 2015;15(13):2332–41.
Kinkead RA, Elliott CT, Cannizzo FT, Biolatti B, Mooney MH. Proteomic identification of plasma proteins as markers of growth promoter abuse in cattle. Anal Bioanal Chem. 2015;407(15):4495–507.
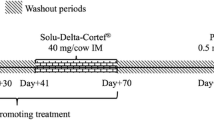
Tarantola M, Schiavone A, Preziuso G, Russo C, Biolatti B, Bergero D. Growth, development and meat science—effects of low doses of dexamethasone on productive traits and meat quality of veal calves. Anim Sci. 2004;79(1):93–8.
Biancotto G, Stella R, Pozza G, Stefani A, Lega F, Angeletti R. Sub-therapeutic treatments of bulls with dexamethasone: direct and indirect markers of treatment. Food Addit Contam Part A: Chem Anal Control Expo Risk Assess. 2013;30(3):430–42.
Gottardo F, Brscic M, Pozza G, Ossensi C, Contiero B, Marin A, et al. Administration of dexamethasone per os in finishing bulls. I. Effects on productive traits, meat quality and cattle behaviour as indicator of welfare. Animal. 2008;2(7):1073–9.
Stella R, Biancotto G, Krogh M, Angeletti R, Pozza G, Sorgato MC, et al. Protein expression changes in skeletal muscle in response to growth promoter abuse in beef cattle. J Proteome Res. 2011;10(6):2744–57.
Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes (86/609/EEC). Off J Eur Commun. 1986;L358:1–28.
Directive 2010/63/EU of the European Parliament and of the council on the protection of animals used for scientific purposes. Off J Eur Commun. 2010;L276:33–79.
Ciccimaro E, Blair IA. Stable-isotope dilution LC–MS for quantitative biomarker analysis. Bioanalysis. 2010;2(2):311–41.
Kirkpatrick DS, Gerber SA, Gygi SP. The absolute quantification strategy: a general procedure for the quantification of proteins and post-translational modifications. Methods. 2005;5(3):265–73.
Kettenbach AN, Rush J, Gerber SA. Absolute quantification of protein and posttranslational modification abundance with stable isotope-labeled synthetic peptides. Nat Protoc. 2011;6(2):175–86.
Holman SW, Sims PFG, Eyers CE. The use of selected reaction monitoring in quantitative proteomics. Bioanalysis. 2012;4(14):1763–86.
Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol. 2009;27(7):633–41.
Stella R, Arrigoni G, Biancotto G, Krogh M, Vascellari M, Lega F, et al. Confirmation of protein biomarkers of corticosteroids treatment in veal calves sampled under field conditions. J Proteome Res. 2014;13(4):1794–9.
Vascellari M, Pozza G, Poppi L, Capello K, Angeletti R, Ravarotto L, et al. Evaluation of indirect biomarkers of corticosteroids use as illegal growth promoters in beef cattle. Vet Rec. 2008;163(5):147–52.
Vascellari M, Capello K, Stefani A, Biancotto G, Moro L, Stella R, et al. Evaluation of thymus morphology and serum cortisol concentration as indirect biomarkers to detect low-dose dexamethasone illegal treatment in beef cattle. BMC Vet Res. 2012;8:129.
Acknowledgments
This work was supported by grants from the Regione del Veneto “Nuovi approcci genomici e proteomici per lo screening dei trattamenti con promotori di crescita nel bovino da carne” (D.G.R. n. 2862 of 28.12.2012, project no. C28C13000080001) and from the Italian Ministry of Health (RF-IZPLV-2006-364645). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animals involved in this study were managed in agreement with the European Directive 86/609/EEC regarding the protection of animals used for experimental or other scientific purposes.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 346 kb)
Rights and permissions
About this article
Cite this article
Stella, R., Barrucci, F., Angeletti, R. et al. Targeted proteomics for the indirect detection of dexamethasone treatment in bovines. Anal Bioanal Chem 408, 8343–8353 (2016). https://doi.org/10.1007/s00216-016-9951-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9951-8