Abstract
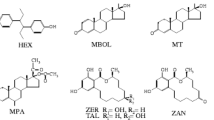
An environmentally friendly method based on hollow-fibre liquid-phase microextraction (HF-LPME) was developed for the extraction of selected estrogenic compounds (i.e. four natural sexual hormones: estrone, 17β-estradiol, 17α-estradiol and estriol; two exoestrogens: 17α-ethynylestradiol and 2-methoxyestradiol; two synthetic stilbenes: dienestrol and hexestrol; and five resorcylic acid lactones: zearalenone, α-zearalanol, β-zearalanol, α-zearalenol and β-zearalenol), from whole cow and semi-skimmed goat milk and whole natural yogurt. After the optimization of the sample preparation procedure, spiked extracts were derivatized to their trimethylsilyl products using N,O-bis(trimethylsilyl)trifluoroacetamide reagent and then analyzed by gas chromatography–tandem mass spectrometry (GC-MS/MS). Once optimum extraction conditions were established (protein precipitation with acetonitrile, extraction and the back-extraction in acetonitrile following the HF-LPME procedure), the method was validated and the calibration range, precision and accuracy were studied. The RSD values for the intra- and inter-day precision of the peak areas were in the range 0.65–9.69 and 1.00–11.47 %, respectively. The determination coefficients were higher than 0.991 for method calibration curves while LOD and LOQ values were between 0.06–2.55 and 0.16–6.11 μg/L for whole cow milk, 0.04–1.70 and 0.11–4.86 μg/L for semi-skimmed goat milk and 0.07–3.73 and 0.23–9.81 μg/L for natural yogurt, respectively. Finally, the accuracy and precision of the method were evaluated, obtaining a value in the range 84 81–119 % and RSD values lower than 20 % in all cases.


Similar content being viewed by others
Abbreviations
- 2-MeOE2 :
-
2-Methoxyestradiol
- DES:
-
Diethylstilbestrol
- DS:
-
Dienestrol
- E1 :
-
Estrone
- E2 :
-
Estradiol
- E3 :
-
Estriol
- EE2 :
-
17α-Ethinylestradiol
- HEX:
-
Hexestrol
- ZAL:
-
Zearalanol
- ZEL:
-
Zearalenol
- ZEN:
-
Zearalenone
References
Rasier G, Toppari J, Parent A-S, Bourguignon J-P. Female sexual maturation and reproduction after prepubertal exposure to estrogens and endocrine disrupting chemicals: a review of rodent and human data. Mol Cell Endocrinol. 2006;254–255:187–201.
Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol. 1998;28:319–61.
Tomšíková H, Aufartová J, Solich P, Nováková L, Sosa-Ferrera Z, Juan Santana-Rodríguez J. High-sensitivity analysis of female-steroid hormones in environmental samples. TrAC Trends Anal Chem. 2012;34:35–58.
Gabet V, Miège C, Bados P, Coquery M. Analysis of estrogens in environmental matrices. TrAC Trends Anal Chem. 2007;26:1113–31.
Alda MJ, Barceló D. Review of analytical methods for the determination of estrogens and progestogens in waste waters. Fresenius J Anal Chem. 2001;371:437–47.
Maruyama K, Oshima T, Ohyama K. Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows. Pediatr Int. 2010;52:33–8.
Pereira PC. Milk nutritional composition and its role in human health. Nutrition. 2013;30:619–27.
Parodi PW. Impact of cows’ milk estrogen on cancer risk. Int Dairy J. 2012;22:3–14.
Ganmaa D, Li X-M, Wang J, Qin L-Q, Wang P-Y, Sato A. Incidence and mortality of testicular and prostatic cancers in relation to world dietary practices. Int J Cancer. 2002;98:262–7.
Eertmans F, Dhooge W, Stuyvaert S, Comhaire F. Endocrine disruptors: effects on male fertility and screening tools for their assessment. Toxicol In Vitro. 2003;17:515–24.
Socas-Rodríguez B, Asensio-Ramos M, Hernández-Borges J, Herrera-Herrera AV, Rodríguez-Delgado MÁ. Chromatographic analysis of natural and synthetic estrogens in milk and dairy products. TrAC Trends Anal Chem. 2013;44:58–77.
Capriotti AL, Cavaliere C, Colapicchioni V, Piovesana S, Samperi R, Laganà A. Analytical strategies based on chromatography-mass spectrometry for the determination of estrogen-mimicking compounds in food. J Chromatogr A. 2013;1313:62–77.
Noppea H, Le Bizecb B, Verheydena K, De Brabandera HF. Novel analytical methods for the determination of steroid hormones in edible matrices. Anal Chim Acta. 2008;611:1–16.
Farlow DW, Xu X, Veenstra TD. Quantitative measurement of endogenous estrogen metabolites, risk-factors for development of breast cancer, in commercial milk products by LC–MS/MS. J Chromatogr B. 2009;877:1327–34.
Sørensen LK, Elbaek TH. Determination of mycotoxins in bovine milk by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;820:183–96.
Chen D, Cao X, Tao Y, Wu Q, Pan Y, Peng D, et al. Development of a liquid chromatography-tandem mass spectrometry with ultrasound-assisted extraction and auto solid-phase clean-up method for the determination of Fusarium toxins in animal derived foods. J Chromatogr A. 2013;1311:21–9.
Xie J, Peng T, Zhu A, He J, Chang Q, Hu X, et al. Multi-residue analysis of veterinary drugs, pesticides and mycotoxins in dairy products by liquid chromatography-tandem mass spectrometry using low-temperature cleanup and solid phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1002:19–29.
Zhao Z, Liu N, Yang L, Deng Y, Wang J, Song S, et al. Multi-mycotoxin analysis of animal feed and animal-derived food using LC-MS/MS system with timed and highly selective reaction monitoring. Anal Bioanal Chem. 2015;407:7359–68.
Xia X, Li X, Ding S, Zhang S, Jiang H, Li J, et al. Ultra-high-pressure liquid chromatography-tandem mass spectrometry for the analysis of six resorcylic acid lactones in bovine milk. J Chromatogr A. 2009;1216:2587–91.
Huang LC, Zheng N, Zheng BQ, Wen F, Cheng JB, Han RW, et al. Simultaneous determination of aflatoxin M1, ochratoxin A, zearalenone and α-zearalenol in milk by UHPLC-MS/MS. Food Chem. 2014;146:242–9.
Wang X, Li P. Rapid screening of mycotoxins in liquid milk and milk powder by automated size-exclusion SPE-UPLC-MS/MS and quantification of matrix effects over the whole chromatographic run. Food Chem. 2015;173:897–904.
Choi MH, Kim KR, Hong JK, Park SJ, Chung BC. Determination of non-steroidal estrogens in breast milk, plasma, urine and hair by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2002;16:2221–8.
Azzouz A, Jurado-Sánchez B, Souhail B, Ballesteros E. Simultaneous determination of 20 pharmacologically active substances in cow’s milk, goat’s milk, and human breast milk by gas chromatography-mass spectrometry. J Agric Food Chem. 2011;59:5125–32.
Courant F, Antignac JP, Laille J, Monteau F, Andre F, Le Bizec B. Exposure assessment of prepubertal children to steroid endocrine disruptors. 2. Determination of steroid hormones in milk, egg, and meat samples. J Agric Food Chem. 2008;56:3176–84.
Courant F, Antignac JP, Maume D, Monteau F, Andre F, Le Bizec B. Determination of naturally occurring oestrogens and androgens in retail samples of milk and eggs. Food Addit Contam. 2007;24:1358–66.
Hartmann S, Lacorn M, Steinhart H. Natural occurrence of steroid hormones in food. Food Chem. 1998;62:7–20.
Su R, Wang X, Xu X, Wang Z, Li D, Zhao X, et al. Application of multiwall carbon nanotubes-based matrix solid phase dispersion extraction for determination of hormones in butter by gas chromatography mass spectrometry. J Chromatogr A. 2011;1218:5047–54.
Xu X, Liang F, Shi J, Zhao X, Liu Z, Wu L, et al. Determination of hormones in milk by hollow fiber-based stirring extraction bar liquid-liquid microextraction gas chromatography mass spectrometry. Anal Chim Acta. 2013;790:39–46.
Zou Y, Zhang Z, Shao X, Chen Y, Wu X, Yang L, et al. Hollow-fiber-supported liquid-phase microextraction using an ionic liquid as the extractant for the pre-concentration of bisphenol A, 17-β-estradiol, estrone and diethylstilbestrol from water samples with HPLC detection. Water Sci Technol. 2014;69:1028–35.
Li H, Jiang Y, Liu Y. Enrichment and determination of trace estradiol in environmental water samples by hollow-fiber liquid-phase microextraction prior to HPLC. J Chromatogr Sci. 2011;49:676–82.
Kanimozhi S, Basheer C, Neveliappan S, Ang K, Xue F, Lee HK. Investigation of bioaccumulation profile of oestrogens in zebrafish liver by hollow fibre protected liquid phase microextraction with gas chromatography-mass spectrometric detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;909:37–41.
Liu Y, Li L, Xu M, Jiang X, Jiang Y. Determination of 2-methoxyestradiol from endometrial cancer patients’ urine by LC-MS/MS with hollow fiber liquid-phase microextraction. Anal Methods. 2013;5:6670–6.
Zha H, Jiang Y, Liu Y, Yun C, Li L. Endogenous estrogen metabolites as biomarkers for endometrial cancer via a novel method of liquid chromatography-mass spectrometry with hollow fiber liquid-phase microextraction. Horm Metab Res. 2015;47:158–64.
Asensio-Ramos M, Ravelo-Pérez LM, González-Curbelo MA, Hernández-Borges J. Liquid phase microextraction applications in food analysis. J Chromatogr A. 2011;1218:7415–37.
Socas-Rodríguez B, Asensio-Ramos M, Hernández-Borges J, Rodríguez-Delgado MÁ. Hollow-fiber liquid-phase microextraction for the determination of natural and synthetic estrogens in milk samples. J Chromatogr A. 2013;1313:175–84.
Socas-Rodríguez B, Asensio-Ramos M, Hernández-Borges J, Rodríguez-Delgado MÁ. Analysis of oestrogenic compounds in dairy products by hollow-fibre liquid-phase microextraction coupled to liquid chromatography. Food Chem. 2014;149:319–25.
Wang P, Xiao Y, Liu W, Wang J, Yang Y. Vortex-assisted hollow fibre liquid-phase microextraction technique combined with high performance liquid chromatography-diode array detection for the determination of oestrogens in milk samples. Food Chem. 2015;172:385–90.
Yang Y, Chen J, Shi YP. Determination of diethylstilbestrol in milk using carbon nanotube-reinforced hollow fiber solid-phase microextraction combined with high-performance liquid chromatography. Talanta. 2012;67:222–8.
Liu M, Li M, Qiu B, Chen X, Chen G. Synthesis and applications of diethylstilbestrol-based molecularly imprinted polymer-coated hollow fiber tube. Anal Chim Acta. 2010;663:33–8.
D’Orazio G, Asensio-Ramos M, Hernández-Borges J, Rodríguez-Delgado MÁ, Fanali S. Evaluation of the combination of a dispersive liquid-liquid microextraction method with micellar electrokinetic chromatography coupled to mass-spectrometry for the determination of estrogenic compounds in milk and yogurt. Electrophoresis. 2015;36:615–25.
Ding W-H, Chiang C-C. Derivatization procedures for the detection of estrogenic chemicals by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:56–63.
European Commission, SANCO/12571/2013. http://ec.europa.eu/food/plant/docs/plant_pesticides_mrl_guidelines_wrkdoc_11945_en.pdf
Hájková K, Pulkrabová J, Schurek J, Hajšlová J, Poustka J, Nápravníková M, et al. Novel approaches to the analysis of steroid estrogens in river sediments. Anal Bioanal Chem. 2007;387:1351–63.
Soliman MA, Pedersen JA, Suffet IH. Rapid gas chromatography–mass spectrometry screening method for human pharmaceuticals, hormones, antioxidants and plasticizers in water. J Chromatogr A. 2004;1029:223–37.
Pacáková V, Loukotková L, Bosáková Z, Stulík K. Analysis for estrogens as environmental pollutants—a review. J Sep Sci. 2009;32:867–82.
Zhao L, Lee HK. Liquid-phase microextraction combined with hollow fiber as a sample preparation technique prior to gas chromatography/mass spectrometry. Anal Chem. 2002;74:2486–92.
Rahman MM, El-Aty A, Shim JH. Matrix enhancement effect: a blessing or a curse for gas chromatography?—a review. Anal Chim Acta. 2013;801:14–21.
Ferrer C, Lozano A, Agüera A, Girón AJ, Fernández-Alba AR. Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables. J Chromatogr A. 2011;1218:7634–9.
Acknowledgments
This work has been supported by the Spanish Ministry of Economy and Competitiveness (project AGL2011-24667). A.V.H.H. wishes to thank the Fundación CajaCanarias for his grant in the Servicios Generales de Apoyo a la Investigación (SEGAI). G. D’Orazio would like to thank the CNR- Institute of Chemical Methodologies, Monterotondo (Rome, Italy) for the support to his PhD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 253 kb)
Rights and permissions
About this article
Cite this article
D’Orazio, G., Hernández-Borges, J., Herrera-Herrera, A.V. et al. Determination of estrogenic compounds in milk and yogurt samples by hollow-fibre liquid-phase microextraction-gas chromatography-triple quadrupole mass spectrometry. Anal Bioanal Chem 408, 7447–7459 (2016). https://doi.org/10.1007/s00216-016-9833-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9833-0