Abstract
Molecular formula assignment is one of the key challenges in processing high-field Fourier transform ion cyclotron resonance mass spectrometric (FT-ICR-MS) datasets. The number of potential solutions for an elemental formula increases exponentially with increasing molecular mass, especially when non-oxygen heteroatoms like N, S or P are included. A method was developed from the chemical perspective and validated using a Suwannee River Fulvic Acid (SRFA) dataset which is dominated by components consisting exclusively of C, H and O (78 % CHO). In order to get information on the application range and robustness of this method, we investigated a FT-ICR-MS dataset which was merged from 18 mine pit lake pore waters and 3 river floodplain soil waters. This dataset contained 50 % CHO and 18 % CHOS on average, whereas the former SRFA dataset contained only 1.5 % CHOS. The mass calculator was configured to allow up to five nitrogen atoms and up to one sulphur atom in assigning formulas to mass peaks. More than 50 % multiple-formula assignments were found for peaks with masses > 650 Da. Based on DBE − O frequency diagrams, many CHO, CHOS1, CHON1 and CHON1S1 molecular series were ultimately assigned to many m/z and considered to be reliable solutions. The unequivocal data pool could thus be enlarged by 523 (6.8 %) CHOS1 components. In contrast to the method validation with CHO-rich SRFA, validation with sulphur-rich pit lake samples showed that formulas with a higher number of non-oxygen heteroatoms can be more reliable assignments in many cases. As an example: CHOS molecular series were reliable and the CHO classes were unreliable amongst other molecular classes in many multiple-formula assignments from the sulphur-rich pit lake samples.

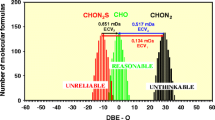
An exemplary frequency versus DBE − O diagram. CHOS components but not CHO (and not CHON2 or CHON2S) components were considered here reliable





Similar content being viewed by others
References
Stenson AC, Landing WM, Marshall AG, Cooper WT. Ionization and fragmentation of humic substances in electrospray ionization Fourier transform-ion cyclotron resonance mass spectrometry. Anal Chem. 2002;74:4397–409.
Stenson AC, Marshall AG, Cooper WT. Exact masses and chemical formulas of individual Suwannee River fulvic acids from ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectra. Anal Chem. 2003;75:1275–84.
Kujawinski EB, Del Vecchio R, Blough NV, Klein GC, Marshall AG. Probing molecular-level transformations of dissolved organic matter: insights on photochemical degradation and protozoan modification of DOM from electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Mar Chem. 2004;92:23–37.
Koch B, Witt M, Engbrodt R, Dittmar T, Kattner G. Molecular formulae of marine and terrigenous dissolved organic matter detected by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Geochim Cosmochim Acta. 2005;69:3299–308.
Reemtsma T, These A, Venkatachari P, Xia XY, Hopke PK, Springer A, et al. Identification of fulvic acids and sulfated and nitrated analogues in atmospheric aerosol by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal Chem. 2006;78:8299–304.
Grannas AM, Hockaday WC, Hatcher PG, Thompson LG, Mosley-Thompson E. New revelations on the nature of organic matter in ice cores. J Geophys Res. 2006;111:(D4).
Hertkorn N, Benner R, Frommberger M, Schmitt-Kopplin P, Witt M, Kaiser K, et al. Characterization of a major refractory component of marine dissolved organic matter. Geochim Cosmochim Acta. 2006;70:2990–3010.
Hertkorn N, Frommberger M, Witt M, Koch BP, Schmitt-Kopplin P, Perdue EM. Natural organic matter and the event horizon of mass spectrometry. Anal Chem. 2008;80:8908–19.
Yassine MM, Harir M, Dabek-Zlotorzynska E, Schmitt-Kopplin P. Structural characterization of organic aerosol using Fourier transform ion cyclotron resonance mass spectrometry: aromaticity equivalent approach. Rapid Commun Mass Spectrom. 2014;28:2445–54.
Marshall AG, Blakney GT, Chen T, Kaiser NK, McKenna AM, Rodgers RP, et al. Mass resolution and mass accuracy: how much is enough? Mass Spectrom. 2013;2(S0009):1.
Koch BP, Dittmar T, Witt M, Kattner G. Fundamentals of molecular formula assignment to ultrahigh resolution mass data of natural organic matter. Anal Chem. 2007;79:1758–63.
Ohno T, Ohno PE. Influence of heteroatom pre-selection on the molecular formula assignment of soil organic matter components determined by ultrahigh resolution mass spectrometry. Anal Bioanal Chem. 2013;405:3299–306.
Kujawinski EB, Longnecker K, Blough NV, Del Vecchio R, Finlay L, Kitner JB, et al. Identification of possible source markers in marine dissolved organic matter using ultrahigh resolution mass spectrometry. Geochim Cosmochim Acta. 2009;73:4384–99.
Ohno T, He Z, Sleighter RL, Honeycutt CW, Hatcher PG. Ultrahigh resolution mass spectrometry and indicator species analysis to identify marker components of soil- and plant biomass-derived organic matter fractions. Environ Sci Technol. 2010;44:8594–600.
Kunenkov EV, Kononikhin AS, Perminova IV, Hertkorn N, Gaspar A, Schmitt-Kopplin P, et al. Total mass difference statistics algorithm: a new approach to identification of high-mass building blocks in electrospray ionization Fourier transform ion cyclotron mass spectrometry data of natural organic matter. Anal Chem. 2009;81:10106–15.
Grinhut T, Lansky D, Gaspar A, Hertkorn N, Schmitt-Kopplin P, Hadar Y, et al. Novel software for data analysis of Fourier transform ion cyclotron resonance mass spectra applied to natural organic matter. Rapid Commun Mass Spectrom. 2010;24:2831–7.
Roach PJ, Laskin J, Laskin A. Higher-order mass defect analysis for mass spectra of complex organic mixtures. Anal Chem. 2011;83:4924–9.
Tziotis D, Hertkorn N, Schmitt-Kopplin P. Kendrick-analogous network visualisation of ion cyclotron resonance Fourier transform mass spectra: improved options for the assignment of elemental compositions and the classification of organic molecular complexity. Eur J Mass Spectrom. 2011;17:415–21.
Kilgour DPA, Mackay CL, Langridge-Smith PRR, O’Connor PB. Appropriate degree of trust: deriving confidence metrics for automatic peak assignment in high-resolution mass spectrometry. Anal Chem. 2012;84:7431–5.
Herzsprung P, Hertkorn N, von Tümpling W, Harir M, Friese K, Schmitt-Kopplin P. Understanding molecular formula assignment of Fourier transform ion cyclotron resonance mass spectrometry data of natural organic matter from a chemical point of view. Anal Bioanal Chem. 2014;406:7977–87.
Herzsprung P, Hertkorn N, Friese K, Schmitt-Kopplin P. Photochemical degradation of natural organic sulfur compounds (CHOS) from iron-rich mine pit lake pore waters-an initial understanding from evaluation of single-elemental formulae using ultra-high-resolution mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:2909–024.
Kotelo LG. Characterising the acid mine drainage potential of fine coal wastes. PHD thesis, University of Cape Town. 2013.
Geller W, Klapper H, Schultze M. Natural and anthropogenic sulfuric acidification of lakes. In: Geller W, Klapper H, Salomons W, editors. Acid mining lakes—acid mine drainage, limnology and reclamation. Berlin, Germany: Springer; 1998. p. 3–14. Environ. Sci. Series.
Dittmar T, Koch B, Hertkorn N, Kattner G. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol Oceanogr Methods. 2008;6:230–5.
Acknowledgments
We thank two anonymous reviewers for very constructive comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Herzsprung, P., Hertkorn, N., von Tümpling, W. et al. Molecular formula assignment for dissolved organic matter (DOM) using high-field FT-ICR-MS: chemical perspective and validation of sulphur-rich organic components (CHOS) in pit lake samples. Anal Bioanal Chem 408, 2461–2469 (2016). https://doi.org/10.1007/s00216-016-9341-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9341-2