Abstract
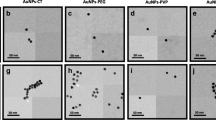
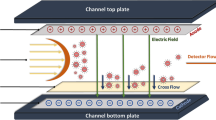
Capillary electrophoresis (CE) is considered as a versatile technique in the size-based separation and speciation of nanomaterials. The electrophoretic mobility is determined by charge and size of an analyte which are affected by the surface composition of nanomaterials. Size-dependent differential electrophoretic mobility is used as a mechanism for size-based separation of nanoparticles. Understanding the effect of surface chemistry on the electrophoretic mobility of nanomaterials in CE is critical in obtaining accurate results in retention-based size calculation. A suite of gold nanoparticles (NPs) varied in sizes with different coatings, including citric acid (CA), lipoic acid (LA), tannic acid (TA), polyvinylpyrrolidone (PVP), polyethylene glycol (PEG), branched polyethyleneimine (BPEI), and bovine serum albumin (BSA), were selected to evaluate their impact to the migration pattern of gold NPs. Additionally, surface-coated gold NPs dispersed in Suwannee River humic acid (SRHA) solution and fetal bovine serum (FBS) were used to investigate the matrix effect. It was found that the correlation between NP size and relative electrophoretic mobility is highly dependent on the capping agents. The matrix component in the SRHA solution only exhibited limited influence to the migration of NPs while electrophoretic behaviors were drastically altered in the presence of FBS matrix.





Similar content being viewed by others
References
Stebounova LV, Morgan H, Grassian VH, Brenner S. Health and safety implications of occupational exposure to engineered nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(3):310–21.
Nyström AM, Fadeel B. Safety assessment of nanomaterials: implications for nanomedicine. J Control Release. 2012;161(2):403–8.
Dhawan A, Sharma V. Toxicity assessment of nanomaterials: methods and challenges. Anal Bioanal Chem. 2010;398(2):589–605.
Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–8.
Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4(1):26–49.
Calzolai L, Gilliland D, Rossi F. Measuring nanoparticles size distribution in food and consumer products: a review. Food Addit Contam Part A. 2012;29(8):1183–93.
von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ, et al. Analysis of engineered nanomaterials in complex matrices (environment and biota): general considerations and conceptual case studies. Environ Toxicol Chem. 2012;31(1):32–49.
Jenkins SV, Qu H, Mudalige T, Ingle TM, Wang R, Wang F, et al. Rapid determination of plasmonic nanoparticle agglomeration status in blood. Biomaterials. 2015;51:226–37.
Anderson W, Kozak D, Coleman VA, Jämting ÅK, Trau M. A comparative study of submicron particle sizing platforms: accuracy, precision and resolution analysis of polydisperse particle size distributions. J Colloid Interface Sci. 2013;405:322–30.
Zhou X-X, Liu R, Liu J-F. Rapid chromatographic separation of dissoluble Ag(I) and silver-containing nanoparticles of 1–100 nanometer in antibacterial products and environmental waters. Environ Sci Technol. 2014;48(24):14516–24.
Soto-Alvaredo J, Montes-Bayón M, Bettmer J. Speciation of silver nanoparticles and silver(I) by reversed-phase liquid chromatography coupled to ICPMS. Anal Chem. 2013;85(3):1316–21. doi:10.1021/ac302851d.
Mudalige TK, Qu H, Linder SW. Asymmetric flow-field flow fractionation hyphenated ICP-MS as an alternative to cloud point extraction for quantification of silver nanoparticles and silver speciation: application for nanoparticles with a protein corona. Anal Chem. 2015;87(14):7395–401.
Qu H, Mudalige TK, Linder SW. Capillary electrophoresis coupled with inductively coupled mass spectrometry as an alternative to cloud point extraction based methods for rapid quantification of silver ions and surface coated silver nanoparticles. J Chromatogr A. 2016;1429:348–53.
Schmidt B, Loeschner K, Hadrup N, Mortensen A, Sloth JJ, Bender Koch C, et al. Quantitative characterization of gold nanoparticles by field-flow fractionation coupled online with light scattering detection and inductively coupled plasma mass spectrometry. Anal Chem. 2011;83(7):2461–8.
Mudalige TK, Qu H, Linder SW. An improved methodology of asymmetric flow field flow fractionation hyphenated with inductively coupled mass spectrometry for the determination of size distribution of gold nanoparticles in dietary supplements. J Chromatogr A. 2015;1420:92–7.
Gray EP, Bruton TA, Higgins CP, Halden RU, Westerhoff P, Ranville JF. Analysis of gold nanoparticle mixtures: a comparison of hydrodynamic chromatography (HDC) and asymmetrical flow field-flow fractionation (AF4) coupled to ICP-MS. J Anal At Spectrom. 2012;27(9):1532–9.
Giddings JC. Field-flow fractionation: analysis of macromolecular, colloidal, and particulate materials. Science. 1993;260(5113):1456–65.
Wahlund K-G. Flow field-flow fractionation: critical overview. J Chromatogr A. 2013;1287:97–112.
Gigault J, Pettibone JM, Schmitt C, Hackley VA. Rational strategy for characterization of nanoscale particles by asymmetric-flow field flow fractionation: a tutorial. Anal Chim Acta. 2014;809:9–24.
Gigault J, Hackley VA. Observation of size-independent effects in nanoparticle retention behavior during asymmetric-flow field-flow fractionation. Anal Bioanal Chem. 2013;405(19):6251–8.
Qu H, Mudalige TK, Linder SW. Capillary electrophoresis/inductively-coupled plasma-mass spectrometry: development and optimization of a high resolution analytical tool for the size-based characterization of nanomaterials in dietary supplements. Anal Chem. 2014;86(23):11620–7.
Ikuta N, Sakamoto H, Yamada Y, Hirokawa T. Numerical simulation for capillary electrophoresis: II. Relaxation effect of potential gradient in capillary zone electrophoresis. J Chromatogr A. 1999;838(1–2):19–29.
Martin MN, Allen AJ, MacCuspie RI, Hackley VA. Dissolution, agglomerate morphology, and stability limits of protein-coated silver nanoparticles. Langmuir. 2014;30(38):11442–52.
Stankus DP, Lohse SE, Hutchison JE, Nason JA. Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ Sci Technol. 2011;45(8):3238–44.
Li X, Lenhart JJ. Aggregation and dissolution of silver nanoparticles in natural surface water. Environ Sci Technol. 2012;46(10):5378–86.
Louie SM, Spielman-Sun ER, Small MJ, Tilton RD, Lowry GV. Correlation of the physicochemical properties of natural organic matter samples from different sources to their effects on gold nanoparticle aggregation in monovalent electrolyte. Environ Sci Technol. 2015;49(4):2188–98.
Qu H, Mudalige TK, Linder SW. Arsenic speciation in rice by capillary electrophoresis/inductively coupled plasma mass spectrometry: enzyme-assisted water-phase microwave digestion. J Agric Food Chem. 2015;63(12):3153–60.
Henry DC. The cataphoresis of suspended particles. Part I. Equ Cataphoresis Proc Roy Soc A. 1931;133(821):106–29.
Doane TL, Chuang C-H, Hill RJ, Burda C. Nanoparticle ζ-potentials. Acc Chem Res. 2012;45(3):317–26.
Schnabel U, Fischer C-H, Kenndler E. Characterization of colloidal gold nanoparticles according to size by capillary zone electrophoresis. J Microcolumn Sep. 1997;9(7):529–34.
Liu FK, Wei GT. Adding sodium dodecylsulfate to the running electrolyte enhances the separation of gold nanoparticles by capillary electrophoresis. Anal Chim Acta. 2004;510(1):77–83.
Soares DM, Gomes WE, Tenan MA. Sodium dodecyl sulfate adsorbed monolayers on gold electrodes. Langmuir. 2007;23(8):4383–8.
Burgess I, Jeffrey CA, Cai X, Szymanski G, Galus Z, Lipkowski J. Direct visualization of the potential-controlled transformation of hemimicellar aggregates of dodecyl sulfate into a condensed monolayer at the Au(111) electrode surface. Langmuir. 1999;15(8):2607–16.
Chauhan S, Sharma K. Effect of temperature and additives on the critical micelle concentration and thermodynamics of micelle formation of sodium dodecyl benzene sulfonate and dodecyltrimethylammonium bromide in aqueous solution: a conductometric study. J Chem Thermodyn. 2014;71:205–11.
Tsai D-H, DelRio FW, Keene AM, Tyner KM, MacCuspie RI, Cho TJ, et al. Adsorption and conformation of serum albumin protein on gold nanoparticles investigated using dimensional measurements and in situ spectroscopic methods. Langmuir. 2011;27(6):2464–77.
Brewer SH, Glomm WR, Johnson MC, Knag MK, Franzen S. Probing BSA binding to citrate-coated gold nanoparticles and surfaces. Langmuir. 2005;21(20):9303–7.
Prapainop K, Witter DP, Wentworth P. A chemical approach for cell-specific targeting of nanomaterials: small-molecule-initiated misfolding of nanoparticle corona proteins. J Am Chem Soc. 2012;134(9):4100–3.
Acknowledgments
These studies were conducted using the Nanotechnology Core Facility (NanoCore) located on the US Food and Drug Administration’s Jefferson Laboratories campus (Jefferson, AR), which houses the FDA National Center for Toxicological Research and the FDA Office of Regulatory Affairs/Arkansas Regional Laboratory. We thank Patrick Sisco, Crystal Ford, Jin-Hee Lim, Yasith Nanayakkara, Venu Gopal Bairi, and Nuwan Kothalawala for their valuable comments on the draft manuscript. This project was supported in part by an appointment to the Research Participation Program at the Office of Regulatory Affairs/Arkansas Regional Laboratory, US Food and Drug Administration, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and FDA. The views expressed in this document are those of the authors and should not be interpreted as the official opinion or policy of the US Food and Drug Administration, Department of Health and Human Services, or any other agency or component of the US government. The mention of trade names, commercial products, or organizations is for clarification of the methods used and should not be interpreted as an endorsement of a product or manufacturer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 251 kb)
Rights and permissions
About this article
Cite this article
Qu, H., Linder, S.W. & Mudalige, T.K. Surface coating and matrix effect on the electrophoretic mobility of gold nanoparticles: a capillary electrophoresis-inductively coupled plasma mass spectrometry study. Anal Bioanal Chem 409, 979–988 (2017). https://doi.org/10.1007/s00216-016-0012-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0012-0