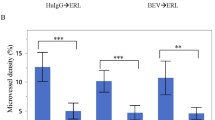
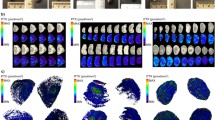
Abstract
The spatial distribution of an anticancer drug and its intended target within a tumor plays a major role on determining how effective the drug can be at tackling the tumor. This study provides data regarding the lateral distribution of sunitinib, an oral antiangiogenic receptor tyrosine kinase inhibitor using an in vitro animal model as well as an in vitro experimental model that involved deposition of a solution of sunitinib onto tissue sections. All tumor sections were analyzed by matrix-assisted laser desorption/ionization mass spectrometry imaging and compared with subsequent histology staining. Six tumors at four different time points after commencement of in vivo sunitinib treatment were examined to observe the patterns of drug uptake. The levels of sunitinib present in in vivo treated tumor sections increased continuously until day 7, but a decrease was observed at day 10. Furthermore, the in vitro experimental model was adjustable to produce a drug level similar to that obtained in the in vivo model experiments. The distribution of sunitinib in tissue sections treated in vitro appeared to agree with the histological structure of tumors, suggesting that this approach may be useful for testing drug update.





Similar content being viewed by others
References
Siegel R, Desantis C, Jemal A (2014) Colorectal cancer statistics, 2014. CA: Cancer J Clin 64(2):104–117. doi:10.3322/caac.21220
Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E (2014) European cancer mortality predictions for the year 2014. Ann Oncol: Off J Eur Soc Med Oncol / ESMO. doi:10.1093/annonc/mdu138
Durko L, Malecka-Panas E (2014) Lifestyle modifications and colorectal cancer. Curr Color Cancer Rep 1(10):45–54
Douillard JY, Sobrero A, Carnaghi C, Comella P, Diaz-Rubio E, Santoro A, Van Cutsem E (2003) Metastatic colorectal cancer: integrating irinotecan into combination and sequential chemotherapy. Ann Oncol: Off J Eur Soc Med Oncol / ESMO 14(Suppl 2):ii7–ii12
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts S (2006) Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol : Off J Am Soc Clin Oncol 24(21):3347–3353. doi:10.1200/jco.2006.06.1317
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol : Off J Am Soc Clin Oncol 22(2):229–237. doi:10.1200/jco.2004.05.113
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Yamada Y, Takahari D, Matsumoto H, Baba H, Nakamura M, Yoshida K, Yoshida M, Iwamoto S, Shimada K, Komatsu Y, Sasaki Y, Satoh T, Takahashi K, Mishima H, Muro K, Watanabe M, Sakata Y, Morita S, Shimada Y, Sugihara K (2013) Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. lancet Oncol 14(13):1278–1286. doi:10.1016/s1470-2045(13)70490-x
Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D (2003) Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest 111(9):1287–1295. doi:10.1172/jci17929
Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P (2004) Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J: Off Publ Fed Am Soc Exp Biol 18(2):338–340. doi:10.1096/fj.03-0271fje
Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Ka M, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD (2006) Sunitinib in patients with metastatic renal cell carcinoma. JAMA : J Am Med Assoc 295(21):2516–2524
George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, Harmon CS, Law CN, Morgan JA, Ray-Coquard I, Tassell V, Cohen DP, Demetri GD (2009) Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer (Oxford, Engl : 1990) 45(11):1959–1968. doi:10.1016/j.ejca.2009.02.011
George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, Harmon CS, Law CNJ, Ja M, Ray-Coquard I, Tassell V, Cohen DP, Demetri GD (2009) Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 45(11):1959–1968
Kulke MH, Bendell J, Kvols L, Picus J, Pommier R, Yao J (2011) Evolving diagnostic and treatment strategies for pancreatic neuroendocrine tumors. J Hematol Oncol 4:29. doi:10.1186/1756-8722-4-29
Norden AD, Drappatz J, Wen PY (2007) Targeted drug therapy for meningiomas. Neurosurg Focus 23(4):E12. doi:10.3171/foc-07/10/e12
Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ (2010) Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 66(2):357–371
Novello S, Scagliotti GV, Rosell R, Ma S, Brahmer J, Atkins J, Pallares C, Burgess R, Tye L, Selaru P, Wang E, Chao R, Govindan R (2009) Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br J Cancer 101(9):1543–1548
Saltz LB, Rosen LS, Marshall JL, Belt RJ, Hurwitz HI, Eckhardt SG, Bergsland EK, Haller DG, Lockhart AC, Rocha Lima CM, Huang X, DePrimo SE, Chow-Maneval E, Chao RC, Lenz HJ (2007) Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol: Off J Am Soc Clin Oncol 25(30):4793–4799. doi:10.1200/jco.2007.12.8637
Moss DM, Siccardi M (2014) Optimising nanomedicine pharmacokinetics using PBPK modelling. Br J Pharmacol. doi:10.1111/bph.12604
Chaurand P, Schwartz SA, Reyzer ML, Caprioli RM (2005) Imaging mass spectrometry: principles and potentials. Toxicol Pathol 33(1):92–101
Végvári Á, Fehniger TE, Rezeli M, Döme B, Jansson B, Welinder C, Marko-Varga G (2013) Experimental models to study drug distributions in tissue using MALDI mass spectrometry imaging. J Proteome Res 12(12):5626–5633
Troendle FJ, Reddick CD, Yost RA (1999) Detection of pharmaceutical compounds in tissue by matrix-assisted laser desorption/ionization and laser desorption/chemical ionization tandem mass spectrometry with a quadrupole ion trap. J Am Soc Mass Spectrom 10(12):1315–1321
Reyzer ML, Hsieh YS, Ng K, Korfmacher WA, Caprioli RM (2003) Direct analysis of drug candidates in tissue by matrix-assisted laser desorption/ionization mass spectrometry. J Mass Spectrom 38(10):1081–1092. doi:10.1002/jms.525
Rohner TC, Staab D, Stoeckli M (2005) MALDI mass spectrometric imaging of biological tissue sections. Mech Ageing Dev 126(1):177–185. doi:10.1016/j.mad.2004.09.032
Hsieh Y, Chen J, Korfmacher WA (2007) Mapping pharmaceuticals in tissues using MALDI imaging mass spectrometry. J Pharmacol Toxicol Methods 55(2):193–200
Prideaux B, Stoeckli M (2012) Mass spectrometry imaging for drug distribution studies. J Proteome 75(16):4999–5013. doi:10.1016/j.jprot.2012.07.028
Källback P, Shariatgorji M, Nilsson A, Andrén PE (2012) Novel mass spectrometry imaging software assisting labeled normalization and quantitation of drugs and neuropeptides directly in tissue sections. J Proteome 75(16):4941–4951. doi:10.1016/j.jprot.2012.07.034
Deininger SO, Cornett DS, Paape R, Becker M, Pineau C, Rauser S, Walch A, Wolski E (2011) Normalization in MALDI-TOF imaging datasets of proteins: practical considerations. Anal Bioanal Chem 401(1):167–181. doi:10.1007/s00216-011-4929-z
Pirman DA, Yost RA (2011) Quantitative tandem mass spectrometric imaging of endogenous acetyl-L-carnitine from piglet brain tissue using an internal standard. Anal Chem 83(22):8575–8581. doi:10.1021/ac201949b
Takai N, Tanaka Y, Inazawa K, Saji H (2012) Quantitative analysis of pharmaceutical drug distribution in multiple organs by imaging mass spectrometry. Rapid Commun Mass Spectrom: RCM 26(13):1549–1556. doi:10.1002/rcm.6256
Hamm G, Bonnel D, Legouffe R, Pamelard F, Delbos JM, Bouzom F, Stauber J (2012) Quantitative mass spectrometry imaging of propranolol and olanzapine using tissue extinction calculation as normalization factor. J Proteomics 75(16):4952–4961. doi:10.1016/j.jprot.2012.07.035
Groseclose MR, Castellino S (2013) A mimetic tissue model for the quantification of drug distributions by MALDI imaging mass spectrometry. Anal Chem 85(21):10099–10106. doi:10.1021/ac400892z
Pirman DA, Reich RF, Kiss A, Heeren RM, Yost RA (2013) Quantitative MALDI tandem mass spectrometric imaging of cocaine from brain tissue with a deuterated internal standard. Anal Chem 85(2):1081–1089. doi:10.1021/ac302960j
Takai N, Tanaka Y, Saji H (2014) Quantification of small molecule drugs in biological tissue sections by imaging mass spectrometry using surrogate tissue-based calibration standards. Mass Spectrom (Tokyo, Japan) 3(1):A0025. doi:10.5702/massspectrometry.A0025
Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P (1995) Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest 96(4):1698–1705. doi:10.1172/jci118214
Manea M, Leurs U, Orban E, Baranyai Z, Ohlschlager P, Marquardt A, Schulcz A, Tejeda M, Kapuvari B, Tovari J, Mezo G (2011) Enhanced enzymatic stability and antitumor activity of daunorubicin-GnRH-III bioconjugates modified in position 4. Bioconjug Chem 22(7):1320–1329. doi:10.1021/bc100547p
Dome B, Paku S, Somlai B, Timar J (2002) Vascularization of cutaneous melanoma involves vessel co-option and has clinical significance. J Pathol 197(3):355–362. doi:10.1002/path.1124
Fehniger TE, Végvári Á, Rezeli M, Prikk K, Ross P, Dahlbäck M, Edula G, Sepper R, Marko-Varga G (2011) Direct demonstration of tissue uptake of an inhaled drug: proof-of-principle study using matrix-assisted laser desorption ionization mass spectrometry imaging. Anal Chem 83(21):8329–8336
Kuh H, Jang S, Wientjes M (1999) Determinants of Paclitaxel penetration and accumulation in human solid tumor. J Pharmacol Exp Ther 290(2):871–880
Annesley TM (2003) Ion suppression in mass spectrometry. Clin Chem 49(7):1041–1044
Fujimura Y, Miura D (2014) MALDI mass spectrometry imaging for visualizing in situ metabolism of endogenous metabolites and dietary phytochemicals. Metabolites 4(2):319–346
Schwandt A, Wood LS, Rini B, Dreicer R (2009) Management of side effects associated with sunitinib therapy for patients with renal cell carcinoma. Oncol Targets Ther 2:51–61
Acknowledgments
AV is grateful for funding support Innovate Support 2011–03926 from CREATE Health. BD was supported by KTIA AIK 12-1-2013-0041, TÁMOP 424A/1-11-1-2012-0001, OTKA K109626, OTKA K108465, EUREKA_HU_12-1-2012-0057, ÖNB Jubiläumsfondsprojekt Nr. 14043, and the Vienna Fund for Innovative Interdisciplinary Cancer Research. JT was supported by OTKA K84173 and INNO 08-3-2009-0248 (2010).
Conflict of interest
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Mass Spectrometry Imaging with guest editors Andreas Römpp and Uwe Karst.
Rights and permissions
About this article
Cite this article
Connell, J.J., Sugihara, Y., Török, S. et al. Localization of sunitinib in in vivo animal and in vitro experimental models by MALDI mass spectrometry imaging. Anal Bioanal Chem 407, 2245–2253 (2015). https://doi.org/10.1007/s00216-014-8350-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8350-2