Abstract
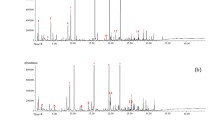
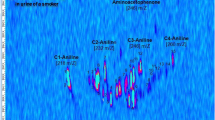
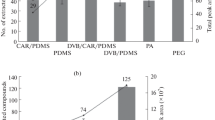
Autism spectrum disorders (ASDs) are a group of neurodevelopmental disorders which have a severe life-long effect on behavior and social functioning, and which are associated with metabolic abnormalities. Their diagnosis is on the basis of behavioral and developmental signs usually detected before three years of age, and there is no reliable biological marker. The objective of this study was to establish the volatile urinary metabolomic profiles of 24 autistic children and 21 healthy children (control group) to investigate volatile organic compounds (VOCs) as potential biomarkers for ASDs. Solid-phase microextraction (SPME) using DVB/CAR/PDMS sorbent coupled with gas chromatography–mass spectrometry was used to obtain the metabolomic information patterns. Urine samples were analyzed under both acid and alkaline pH, to profile a range of urinary components with different physicochemical properties. Multivariate statistics techniques were applied to bioanalytical data to visualize clusters of cases and to detect the VOCs able to differentiate autistic patients from healthy children. In particular, orthogonal projections to latent structures discriminant analysis (OPLS-DA) achieved very good separation between autistic and control groups under both acidic and alkaline pH, identifying discriminating metabolites. Among these, 3-methyl-cyclopentanone, 3-methyl-butanal, 2-methyl-butanal, and hexane under acid conditions, and 2-methyl-pyrazine, 2,3-dimethyl-pyrazine, and isoxazolo under alkaline pH had statistically higher levels in urine samples from autistic children than from the control group. Further investigation with a higher number of patients should be performed to outline the metabolic origins of these variables, define a possible association with ASDs, and verify the usefulness of these variables for early-stage diagnosis.

ᅟ






Similar content being viewed by others
References
Angley M, Young R, Ellis D, Chan W, McKinnon R (2007) Children and autism–part 1–recognition and pharmacological management. Aust Fam Physician 36:741–744
Johnson CP, Myers SM (2007) Identification and evaluation of children with autism spectrum disorders. Pediatrics 120:1183–1215
Herb B (2012) Autism. Nature 491:S1–S48
Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK (2010) Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res 9:2996–3004
London EA, Etzel RA (2000) The environment as an etiologic factor in autism: a new direction for research. Environ Health Perspect 108:401–404
Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL (2004) White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychol 55:323–326
Kałużna-Czaplińska J, Blaszczyk S (2012) The level of arabinitol in autistic children after probiotic therapy. Nutrition 28:124–126
Ming X, Stein TP, Barnes V, Rhodes N, Guo L (2012) Metabolic perturbance in autism spectrum disorders: a metabolomics study. J Proteome Res 11:5856–5862
Perry TL, Hansen S, Christie RG (1978) Amino compounds and organic acids in CSF, plasma, and urine of autistic children. Biol Psychiatry 13:575–586
Winsberg BG, Sverd J, Castells S, Hurwic M, Perel JM (1980) Estimation of monoamine and cyclic-AMP turnover and amino acid concentrations of spinal fluid in autistic children. Neuropediatrics 11:250–255
Rolf LH, Haarmann FY, Grotemeyer KH, Kehrer H (1993) Serotonin and amino acid content in platelets of autistic children. Acta Psychiatr Scand 87:312–316
Tirouvanziam R, Obukhanych TV, Laval J, Aronov PA, Libove R, Banerjee AG, Parker KJ, O’Hara R, Herzenberg LA, Herzenberg LA, Hardan AY (2012) Distinct plasma profile of polar neutral amino acids, leucine, and glutamate in children with autism spectrum disorders. J Autism Dev Disord 42:827–836
Ratajczak HV (2011) Theoretical aspects of autism: biomarkers–a review. J Immunot 8:80–94
Rossignol DA, Frye RE (2012) Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry 17:290–314
Laszlo A, Horvath E, Eck E, Fekete M (1994) Serum serotonin, lactate and pyruvate levels in infantile autistic children. Clin Chim Acta 229:205–207
Bradstreet JJ, Smith S, Baral M, Rossignol DA (2010) Biomarker-guided interventions of clinically relevant conditions associated with autism spectrum disorders and attention deficit hyperactivity disorder. Altern Med Rev 15:15–32
James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80:1611–1617
Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, Gehn E, Loresto M, Mitchell J, Atwood S, Barnhouse S, Lee W (2011) Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr Metab 8:34–65
Gu F, Chauhan V, Kaur K, Brown WT, LaFauci G, Wegiel J, Chauhan A (2013) Alterations in mitochondrial DNA copy number and the activities of electron transport chain complexes and pyruvate dehydrogenase in the frontal cortex from subjects with autism. Transl Psychiatr 3:1–8
Parracho HMRT, Bingham MO, Gibson GR, McCartney AL (2005) Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 54:987–991
de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, Militerni R, Bravaccio C (2010) Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr 51:418–424
Williams BL, Hornig M, Parekh T, Lipkin WI (2012) Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio 3:1–3
De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R (2013) Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE 8:e76993
Wang L, Angley MT, Gerber JP, Sorich MJ (2011) A review of candidate urinary biomarkers for autism spectrum disorder. Biomarkers 16:537–552
Żurawicz E, Kałużna-Czaplińska J, Rynkowski J (2013) Chromatographic methods in the study of autism. Biomed Chromatogr 27:1273–1279
Madsen R, Lundstedt T, Trygg J (2010) Chemometrics in metabolomics–a review in human disease diagnosis. Anal Chim Acta 659:23–33
Mills GA, Walker V (2001) Headspace solid-phase microextraction profiling of volatile compounds in urine: application to metabolic investigations. J Chromatogr B Biomed Sci Appl 753:259–268
Zlatkis A, Brazell RS, Poole CF (1981) The role of organic volatile profiles in clinical diagnosis. Clin Chem 27:789–797
Wahl HG, Hoffmann A, Luft D, Liebich HM (1999) Analysis of volatile organic compounds in human urine by headspace gas chromatography-mass spectrometry with a multipurpose sampler. J Chromatogr A 847:117–125
Shirasu M, Touhara K (2011) The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem 150:257–266
Lechner M, Rieder J (2007) Mass spectrometric profiling of low-molecular-weight volatile compounds–diagnostic potential and latest applications. Curr Med Chem 14:987–995
Banday KM, Pasikanti KK, Chan EC, Singla R, Rao KV, Chauhan VS, Nanda RK (2011) Use of urine volatile organic compounds to discriminate tuberculosis patients from healthy subjects. Anal Chem 83:5526–5534
Silva CL, Passos M, Câmara JS (2011) Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br J Cancer 105:1894–1904
Silva CL, Passos M, Câmara JS (2012) Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers–a powerful strategy for breast cancer diagnosis. Talanta 89:360–368
Pawliszyn J (2009) Handbook of Solid Phase Microextraction. Chemical Industry Press, Beijing
Nicholson JK, Lindon JC (2008) Systems biology: metabonomics. Nature 455:1054–1056
Jackson JE (1991) A users guide to principal components. John Wiley, New York
Barker M, Rayens W (2007) Partial least squares for discrimination. J Chemometr 17:166–173
Trygg J, Wold S (2002) Orthogonal projections to latent structures (O-PLS). J Chemom 16:119–128
Trygg J, Holmes E, Lundstedt T (2007) Chemometrics in metabonomics. J Proteome Res 6:469–479
Broadhurst DI, Kell DB (2006) Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics 2:171–196
Westerhuis JA, Hoefsloot HCJ, Smit S, Vis DJ, Smilde AK, van Velzen EJJ, van Duijnhoven JPM, van Dorsten FA (2008) Assessment of PLSDA cross validation. Metabolomics 4:81–89
Fawcett T (2006) An introduction to ROC analysis. Pattern Recogn Lett 27:861–874
Kataokaa H, Lordb HL, Pawliszynb J (2000) Applications of solid-phase microextraction in food analysis. J Chromatogr A 880:35–62
Zhang Z, Pawliszyn J (1993) Headspace solid-phase microextraction. Anal Chem 65:1843–1852
Deng C, Zhang X, Li N (2004) Investigation of volatile biomarkers in lung cancer blood using solid-phase microextraction and capillary gas chromatography–mass spectrometry. J Chromatogr B 808:269–277
Mochalski P, Wzorek B, Sliwka I, Amann A (2009) Improved pre-concentration and detection methods for volatile sulphur breath constituents. J. Chromatogr B 877:1856–1866
Lindon JC, Nicholson JK, Holmes E, Keun HC, Craig A, Pearce JTM, Bruce SJ, Hardy N, Sansone SA, Antti H, Jonsson P, Daykin C, Navarange M, Beger RD, Verheij ER, Amberg A, Baunsgaard D, Cantor GH, Lehman-McKeeman L, Earll M, Wold S, Johansson E, Haselden JN, Kramer K, Thomas C, Lindberg J, Schuppe-Koistinen I, Wilson ID, Reily MD, Robertson DG, Senn H, Krotzky A, Kochhar S, Powell J, van der Ouderaa F, Plumb R, Schaefer H, Spraul M (2005) worki SMRS (is this right), Summary recommendations for standardization and reporting of metabolic analyses. Nat Biotechnol 23:833–838
Goodacre R, Broadhurst D, Smilde AK, Kristal BS, Baker JD, Beger R, Bessant C, Connor S, Calmani G, Craig A, Ebbels T, Kell DB, Manetti C, Newton J, Paternostro G, Somorjai R, Sjostrom M, Trygg J, Wulfert F (2007) Proposed minimum reporting standards for data analysis in metabolomics. Metabolomics 3:231–241
Worley B, Powers R (2013) Multivariate analysis in metabolomics. Curr Metabolomics 1:92–107
Dieterle F, Ross A, Schlotterbeck G, Senn H (2006) Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. application in 1H NMR metabonomics. Anal Chem 78:4281–4290
Veselkov KA, Vingara LK, Masson P, Robinette SL, Want E, Li JV, Barton RH, Boursier-Neyret C, Walther B, Ebbels TM, Pelczer I, Holmes E, Lindon JC, Nicholson JK (2011) Optimized preprocessing of ultra performance liquid chromatography/mass spectrometry urinary metabolic profiles for improved information recovery. Anal Chem 83:5864–5872
Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikström C, Wold S (2006) Multi- and megavariate data analysis. Basic principles and applications. Appendix, IIth edn. Umetrics AB, Sweden, Umeå
Wiklund S, Johansson E, Sjöstroöm L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J (2008) Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem 80:115–122
Smit BA, Engels WJM, Smit G (2009) Branched chain aldehydes: production and breakdown pathways and relevance for flavour in foods. Appl Microbiol Biotechnol 81:987–999
Chauhan A, Chauhan V, Brown WT, Cohen I (2004) Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin–the antioxidant proteins. Life Sci 75:2539–2549
Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT (1999) Gastrointestinal abnormalities in children with autistic disorder. J Pediatr 135:559–563
Kushak RI, Lauwers GY, Winter HS, Buie TM (2011) Intestinal disaccharidase activity in patients with autism: effect of age, gender, and intestinal inflammation. Autism 15:285–294
Maga JA (1992) Wazines update. Food Rev Znt 8:479–558
Lancker FV, Adams A, De Kimpe N (2010) Formation of pyrazines in Maillard model system of lysine-containing dipeptides”. J Agric Food Chem 58:2470–2478
Nicolotti L, Cordero C, Bicchi C, Rubiolo P, Sgorbini B, Liberto E (2013) Volatile profiling of high quality hazelnuts (Corylus avellana L.): chemical indices of roastin. Food Chem 138:1723–1733
Kim YH (2013) An accurate and reliable analysis of trimethylamine using thermal desorption and gas chromatography-time of flight mass spectrometry. Anal Chim Acta 780:46–54
Mitchell SC, Zhang AQ, Smith RL (2002) Chemical and biological liberation of trimethylamine from food. J Food Comp Anal 15:277–282
Acknowledgment
We thank study subjects and their families for participating in this study, and Dr Spagnuolo, head of the no-profit association “Associazione Irpinia Pianeta Autismo (AIPA)” located in Avellino (Italy), for her valuable support. We would also like to acknowledge Christopher Nardone for reviewing the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 421 kb)
Rights and permissions
About this article
Cite this article
Cozzolino, R., De Magistris, L., Saggese, P. et al. Use of solid-phase microextraction coupled to gas chromatography–mass spectrometry for determination of urinary volatile organic compounds in autistic children compared with healthy controls. Anal Bioanal Chem 406, 4649–4662 (2014). https://doi.org/10.1007/s00216-014-7855-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7855-z