Abstract
Paraoxonase-1 (PON1) and butyrylcholinesterase (BCHE) are natural bioscavengers of organophosphate acetylcholinesterase inhibitors in the human body, which can determine individual sensitivity to organophosphate toxicity. Interindividual differences in activity of PON1 (catalytic bioscavenger) and substrate specificity are strongly associated with the substitution of two amino acids: Leu/Met (L/M) at position 55 (rs854560) and Gln/Arg (Q/R) at position 192 (rs662). In the case of BCHE (stoichiometric bioscavenger) substitution, Ala/Thr (A/T) at position 539 produces the so-called “K-variant” of the enzyme (rs1803274). Threonine allele is often co-inherited with an atypical BCHE allele (rs1799807). The atypical variant of BCHE displays a lower affinity for cholinesterase inhibitors. Genotyping rs662 and rs1803274 single-nucleotide polymorphisms (SNP) by high-resolution melting (HRM) is facilitated by the nucleotide substitution A>G (G>A), which resulted in a changed number of hydrogen bonds in the PCR product and, consequently, shifted T m. In the case of rs854560, genotyping is complicated by the nucleotide substitution T>A, which has no significant effect on the T m of the PCR product. An addition of a small quantity of LL homozygote DNA into the reaction mixture before PCR discriminates the three genotypes by the melt curves due to different amounts of heteroduplexes formed in the LM and MM samples. HRM analysis can be applied for genotyping human rs854560, rs662, and rs1803274 SNPs.

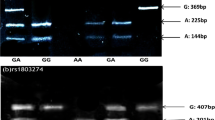
Difference curve pattern of amplicons containing SNP rs1803274



Similar content being viewed by others
References
Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN (2005) Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res 46:1236–1247
Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE (1996) The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet 14:334–336
Kanamori-Kataoka M, Seto Y (2009) Paraoxonase activity against nerve gases measured by capillary electrophoresis and characterization of human serum paraoxonase (PON1) polymorphism in the coding region (Q192R). Anal Biochem 385:94–100
Valiyaveettil M, Alamneh Y, Rezk P, Perkins MW, Sciuto AM, Doctor BP, Nambiar MP (2011) Recombinant paraoxonase 1 protects against sarin and soman toxicity following microinstillation inhalation exposure in guinea pigs. Toxicol Lett 202:203–208
Mackness B, Mackness MI, Arrol S, Turkie W, Durrington PN (1997) Effect of the molecular polymorphisms of human paraoxonase (PON1) on the rate of hydrolysis of paraoxon. Br J Pharmacol 122:265–268
Doctor BP, Saxena A (2005) Bioscavengers for the protection of humans against organophosphate toxicity. Chem Biol Interact 157–158:167–171
Hofmann JN, Keifer MC, Furlong CE, De Roos AJ, Farin FM, Fenske RA, van Belle G, Checkoway H (2009) Serum cholinesterase inhibition in relation to paraoxonase-1 (PON1) status among organophosphate-exposed agricultural pesticide handlers. Environ Health Perspect 117:1402–1408
Albers JW, Garabrant DH, Berent S, Richardson RJ (2010) Paraoxonase status and plasma butyrylcholinesterase activity in chlorpyrifos manufacturing workers. J Expo Sci Environ Epidemiol 20:79–89
Furtado-Alle L, Andrade FA, Nunes K, Mikami LR, Souza RL, Chautard-Freire-Maia EA (2008) Association of variants of the −116 site of the butyrylcholinesterase BCHE gene to enzyme activity and body mass index. Chem Biol Interact 175:115–118
Dimov D, Kanev K, Dimova I (2012) Correlation between butyrylcholinesterase variants and sensitivity to soman toxicity. Acta Biochim Pol 59:313–316
Bartels CF, Jensen FS, Lockridge O, van der Spek AF, Rubinstein HM, Lubrano T, La Du BN (1992) DNA mutation associated with the human butyrylcholinesterase K-variant and its linkage to the atypical variant mutation and other polymorphic sites. Am J Hum Genet 50:1086–1103
Vossen RH, Aten E, Roos A, den Dunnen JT (2009) High-resolution melting analysis (HRMA): more than just sequence variant screening. Hum Mutat 30:860–866
Masson P, Rochu D (2009) Catalytic bioscavengers against toxic esters, an alternative approach for prophylaxis and treatments of poisonings. Acta Nat 1:68–79
Leviev I, Deakin S, James RW (2001) Decreased stability of the M54 isoform of paraoxonase as a contributory factor to variations in human serum paraoxonase concentrations. J Lipid Res 42:528–535
Garin MC, James RW, Dussoix P, Blanché H, Passa P, Froguel P, Ruiz J (1997) Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J Clin Invest 99:62–66
Altamirano CV, Bartels CF, Lockridge O (2000) The butyrylcholinesterase K-variant shows similar cellular protein turnover and quaternary interaction to the wild-type enzyme. J Neurochem 74:869–877
Bartels CF, van der Spek AFL, La Du BN (1990) Two polymorphisms in the noncoding regions of the BCHE gene. Nucleic Acids Res 18:6171
McGuire MC, Nogueira CP, Bartels CF, Lightstone H, Hajra A, Van der Spek AF, Lockridge O, La Du BN (1989) Identification of the structural mutation responsible for the dibucaine-resistant (atypical) variant form of human serum cholinesterase. Proc Natl Acad Sci U S A 86:953–957
Kurdyukov I, Dubrovsky Y, Babakov V, Goncharov N (2012) Investigation of paraoxonase-1 polymorphisms in habitants of Kirov region. Toksikologicheskii vestnik 4:13–18
Agachan B, Yilmaz H, Ergen HA, Karaali ZE, Isbir T (2005) Paraoxonase (PON1) 55 and 192 polymorphism and its effects to oxidant-antioxidant system in Turkish patients with type 2 diabetes mellitus. Physiol Res 54:287–293
Liew M, Pryor R, Palais R, Meadows C, Erali M, Lyon E, Wittwer C (2004) Genotyping of single-nucleotide polymorphisms by high-resolution melting of small amplicons. Clin Chem 50:1156–1164
Palais RA, Liew MA, Wittwer CT (2005) Quantitative heteroduplex analysis for single nucleotide polymorphism genotyping. Anal Biochem 346:167–175
Seipp MT, Herrmann M, Wittwer CT (2010) Automated DNA extraction, quantification, dilution, and PCR preparation for genotyping by high-resolution melting. J Biomol Tech 21:163–166
Acknowledgments
We thank Dr. N.S. Khlebnikova for critically reading the manuscript. This work was supported by the Russian Federal Medical Biological Agency (contract nos. 25.442.12.0 and 25.441.13.0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Analysis of Chemicals Relevant to the Chemical Weapons Convention with guest editors Marc-Michael Blum and R. V. S. Murty Mamidanna.
Rights and permissions
About this article
Cite this article
Kurdyukov, I., Rodionov, G., Radilov, A. et al. Genotyping single-nucleotide polymorphisms of human genes involved in organophosphate detoxification by high-resolution melting. Anal Bioanal Chem 406, 5087–5092 (2014). https://doi.org/10.1007/s00216-014-7734-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7734-7