Abstract
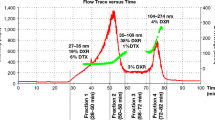
Knowledge about drug retention within colloidal carriers is of uppermost importance particularly if drug targeting is anticipated. The aim of the present study was to evaluate asymmetrical flow field-flow fractionation (AF4) with on-line UV/VIS drug quantification for its suitability to determine both release and transfer of drug from liposomal carriers to a model acceptor phase consisting of large liposomes. The hydrophobic porphyrin 5,10,15,20-tetrakis(4-hydroxyphenyl)21H,23H-porphine (p-THPP), a fluorescent dye with an absorbance maximum in the visible range and structural similarity to the clinically used photosensitizer temoporfin, was used as a model drug, and two types of large liposomes were studied as a potential model acceptor phase. Efficiency of separation of small donor from large acceptor liposomes by AF4 was evaluated in dependence on the injected lipid mass using two different channel geometries. Drug quantification by on-line absorbance measurements was established by comprehensive evaluation of the size-dependent turbidity contribution in on-line UV/VIS detection and by comparison with off-line results obtained for the respective dye-loaded donor formulations (dissolved in methanol). Due to distinct differences in size, the acceptor liposomes (mean diameters ∼300–400 nm) could efficiently be separated from the donor liposomes (mean diameter ∼70 nm) with less than 4 % of p-THPP detected in the overlap region between both vesicle populations. Whereas p-THPP could accurately be determined in the fraction of small vesicles, on-line quantification was impaired in the fraction of the large acceptor liposomes due to the pronounced contribution of turbidity (about 80 % of total UV/VIS extinction signal). The AF4-based release/transfer approach suggested here was found repeatable and robust. The employed combination of AF4 with multi-angle laser light scattering furthermore provided detailed size information of the eluting sample and thus allowed to detect instabilities and/or interactions between the donor and acceptor liposomes. Drug quantification by on-line absorbance measurements was found feasible for the chosen model drug, but careful (re-)evaluation of turbidity effects is crucial for other drug and carrier combinations.









Similar content being viewed by others
References
Shi Y, Porter W, Merdan T, Li LC (2009) Recent advances in intravenous delivery of poorly water-soluble compounds. Expert Opin Drug Del 6(12):1261–1282. doi:10.1517/17425240903307423
Maruyama K (2011) Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev 63(3):161–169
Fahr A, van Hoogevest P, May S, Bergstrand N, Leigh MLS (2005) Transfer of lipophilic drugs between liposomal membranes and biological interfaces: consequences for drug delivery. Eur J Pharm Sci 26(3–4):251–265. doi:10.1016/j.ejps.2005.05.012
Johnston MJW, Semple SC, Klimuk SK, Edwards K, Eisenhardt ML, Leng EC, Karlsson G, Yanko D, Cullis PR (2006) Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Bba-Biomembranes 1758(1):55–64. doi:10.1016/j.bbamem.2006.01.009
Washington C (1990) Drug release from microdisperse systems—a critical-review. Int J Pharm 58(1):1–12. doi:10.1016/0378-5173(90)90280-H
Wallace SJ, Li J, Nation RL, Boyd BJ (2012) Drug release from nanomedicines: selection of appropriate encapsulation and release methodology. Drug Deliv Transl Res 2(4):284–292. doi:10.1007/s13346-012-0064-4
Shabbits JA, Chiu GNC, Mayer LD (2002) Development of an in vitro drug release assay that accurately predicts in vivo drug retention for liposome-based delivery systems. J Control Release 84(3):161–170. doi:10.1016/S0168-3659(02)00294-8
Petersen S, Fahr A, Bunjes H (2010) Flow cytometry as a new approach to investigate drug transfer between lipid particles. Mol Pharmaceut 7(2):350–363. doi:10.1021/Mp900130s
Hefesha H, Loew S, Liu XL, May S, Fahr A (2011) Transfer mechanism of temoporfin between liposomal membranes. J Control Release 150(3):279–286. doi:10.1016/j.jconrel.2010.09.021
Decker C, Steiniger F, Fahr A (2013) Transfer of a lipophilic drug (temoporfin) between small unilamellar liposomes and human plasma proteins: influence of membrane composition on vesicle integrity and release characteristics. J Liposome Res 23(2):154–165. doi:10.3109/08982104.2013.770017
Fraunhofer W, Winter G (2004) The use of asymmetrical flow field-flow fractionation in pharmaceutics and biopharmaceutics. Eur J Pharm Biopharm 58(2):369–383. doi:10.1016/j.ejpb.2004.03.034
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J (2011) Photodynamic therapy of cancer: an update. Ca-Cancer J Clin 61(4):250–281. doi:10.3322/Caac.20114
Schubert R (2003) Liposome preparation by detergent removal. Method Enzymol 367:46–70
Kuntsche J, Decker C, Fahr A (2012) Analysis of liposomes using asymmetrical flow field-flow fractionation: separation conditions and drug/lipid recovery. J Sep Sci 35(15):1993–2001. doi:10.1002/jssc.201200143
Kuntsche J, Freisleben I, Steiniger F, Fahr A (2010) Temoporfin-loaded liposomes: physicochemical characterization. Eur J Pharm Sci 40(4):305–315. doi:10.1016/j.ejps.2010.04.005
Hupfeld S, Moen HH, Ausbacher D, Haas H, Brandl M (2010) Liposome fractionation and size analysis by asymmetrical flow field-flow fractionation/multi-angle light scattering: influence of ionic strength and osmotic pressure of the carrier liquid. Chem Phys Lipids 163(2):141–147. doi:10.1016/j.chemphyslip.2009.10.009
Hupfeld S, Ausbacher D, Brandl M (2009) Asymmetric flow field-flow fractionation of liposomes: 2. Concentration detection and adsorptive loss phenomena. J Sep Sci 32(20):3555–3561. doi:10.1002/jssc.200900292
Hupfeld S, Ausbacher D, Brandl M (2009) Asymmetric flow field-flow fractionation of liposomes: optimization of fractionation variables. J Sep Sci 32(9):1465–1470. doi:10.1002/jssc.200800626
Hupfeld S, Holsaeter AM, Skar M, Frantzen CB, Brandl M (2006) Liposome size analysis by dynamic/static light scattering upon size exclusion-/field flow-fractionation. J Nanosci Nanotechno 6(9–10):3025–3031. doi:10.1166/Jnn.2006.454
Brandl M (2001) Liposomes as drug carriers: a technological approach. Biotechnol Annu Rev 7:59–85
Finsy R (1994) Particle sizing by quasi-elastic light-scattering. Adv Colloid Interfac 52:79–143. doi:10.1016/0001-8686(94)80041-3
Kuntsche J, Klaus K, Steiniger F (2009) Size determinations of colloidal fat emulsions: a comparative study. J Biomed Nanotechnol 5(4):384–395. doi:10.1166/jbn.2009.1047
Stauch O, Schubert R, Savin G, Burchard W (2002) Structure of artificial cytoskeleton containing liposomes in aqueous solution studied by static and dynamic light scattering. Biomacromolecules 3(3):565–578
Holzer M, Barnert S, Momm J, Schubert R (2009) Preparative size exclusion chromatography combined with detergent removal as a versatile tool to prepare unilamellar and spherical liposomes of highly uniform size distribution. J Chromatogr A 1216(31):5838–5848. doi:10.1016/j.chroma.2009.06.023
Chong CS, Colbow K (1976) Light-scattering and turbidity measurements on lipid vesicles. Biochimica Et Biophysica Acta 436(2):260–282. doi:10.1016/0005-2736(76)90192-9
Zattoni A, Melucci D, Torsi G, Reschiglian P (2000) Quantitative analysis in field-flow fractionation using ultraviolet-visible detectors: an experimental design for absolute measurements. J Chromatogr Sci 38(3):122–128
Gregory J (1998) Turbidity and beyond. Filtr Separat 35(1):63–67. doi:10.1016/S0015-1882(97)83117-5
Moon MH, Giddings JC (1993) Size distribution of liposomes by flow field-flow fractionation. J Pharmaceut Biomed 11(10):911–920. doi:10.1016/0731-7085(93)80049-7
Acknowledgments
The authors thank the Phospholipid Research Center Heidelberg (Germany) for the financial support and Rolf Schubert and his group at the University of Freiburg (Institute of Pharmaceutical Technology and Biopharmacy, Freiburg, Germany) for kindly providing the liposomes prepared by detergent removal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Field- and Flow-based Separations with guest editors Gaetane Lespes, Catia Contado, and Bruce Gale.
Rights and permissions
About this article
Cite this article
Hinna, A., Steiniger, F., Hupfeld, S. et al. Asymmetrical flow field-flow fractionation with on-line detection for drug transfer studies: a feasibility study. Anal Bioanal Chem 406, 7827–7839 (2014). https://doi.org/10.1007/s00216-014-7643-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7643-9