Abstract
Silver nucleation on gold has been exploited for signal amplification and has found application in several qualitative and quantitative bio-sensing techniques, thanks to the simplicity of the method and the high sensitivity achieved. Very recently, this technique has been tentatively applied to improve the performance of gold-based immunoassays. In this work, the exploitation of the signal amplification due to silver deposition on gold nanoparticles has been first applied to a competitive lateral flow immunoassay (LFIA). The signal enhancement due to silver allowed us to strongly reduce the amount of the competitor and of specific antibodies employed to build an LF device for measuring ochratoxin A (OTA), thus permitting the attainment of a highly sensitive assessment of OTA contamination, with a sensitivity gain of more than 10-fold compared to the gold-based LFIA that used the same immunoreagents and to all previously reported LFIA for measuring OTA. In addition, a less sensitive “quantitative” LFIA could be established, by suitably tuning competitor and antibody amounts, which was characterized by reproducible and accurate OTA determinations (RSD% 6–12 %, recovery% 82–117 %). The quantitative system allowed a reliable OTA quantification in wines and grape musts at the microgram per liter level requested by the European legislation, as demonstrated by a highly results obtained through the quantitative silver-enhanced LFIA and a reference HPLC-FLD on 30 samples.

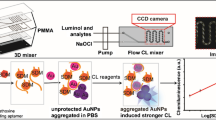
The silver enhanced-Lateral Flow ImmunoAssay: strip development based on gold-nanoparticles occurs, followed by the addition of the enhancing solution, which causes the lines to turn black and become more intense, thus increasing detectability.






Similar content being viewed by others
References
Liu R, Zhang Y, Zhang S, Qiu W, Gao Y (2014) Silver enhancement of gold nanoparticles for biosensing: from qualitative to quantitative. App Spectr Rev 49:121–138, doi: 10.1080/05704928.2013.807817
James TH (1939) The reduction of silver ions by hydroquinone. J Am Chem Soc 61:648–652
Gupta S, Huda S, Kilpatrick PK, Velev OD (2007) Characterization and optimization of gold nanoparticle-based silver-enhanced immunoassays. Anal Chem 79:3810–3820
Yang W, Li XB, Liu GW, Zhang BB, Zhang Y, Kong T, Tang JJ, Li DN, Wang ZA (2011) Colloidal gold probe-based silver enhancement immunochromatographic assay for the rapid detection of abrin-a. Bioasens Bioelectron 26:3710–3713
Linares EM, Kubota LT, Michaelis J, Thalhammer S (2012) Enhancement of the detection limit for lateral flow immunoassays: evaluation and comparison of bioconjugates. J Immunol Met 375:264–270
Anfossi L, Giovannoli C, Giraudi G, Biagioli F, Passini C, Baggiani C (2012) A lateral flow immunoassay for the rapid detection of ochratoxin A in wine and grape must. J Agric Food Chem 60:11491–11497
Nielsen KF, Mogensen JM, Johansen M, Larsen TO, Frisvad JC (2009) Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal Bioana Chem 395:1225–1242
Walker R (2002) Risk assessment of ochratoxin: current views of the European Scientific Committee on Food, the JECFA and the Codex Committee on Food Additives and Contaminants. Adv Exp Med Bio 504:249–255
International Agency of Research on Cancer (1993) IARC monographs on the evaluation of carcinogenic risk to humans, vol no. 56. IARC, Lyon
European commission (2006) Commission Regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Comm L364:5–24
European commission (2010) Commission Regulation (EC) No 105/2010 of 5 February 2010 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards ochratoxin A. Off J Eur Comm L35:7–8
Van Egmond HP, Schothorst RC, Jonker MA (2007) Regulations relating to mycotoxins in food: perspectives in a global and European context. Anal Bioanal Chem 389:147–157
European Mycotoxin Awareness Network (EMAN) Mycotoxins Legislation Worldwide (last updated February 2012) http://services.leatherheadfood.com/eman/FactSheet.aspx?ID=79. Accessed 28 Aug 2013
SCOOP 2002. Report of experts participating in Task 3.2.7, Brussels, Belgium. http://ec.europa.eu/food/fs/scoop/3.2.7_en.pdf. Accessed 28 Aug 2013
Health Canada. Canadian Food and drug regulations. http://www.cfs.gov.hk/english/programme/programme_rafs/files/cfs_news_ras_23_och.pdf Accessed 28 Aug 2013
Krska R, Becalski A, Braekevelt E, Koerner T, Cao XL, Dabeka R, Godefroy S, Lau B, Moisey J, Rawn DF, Scott PM, Wang Z, Forsyth D (2012) Challenges and trends in the determination of selected chemical contaminants and allergens in food. Anal Bioanal Chem 402:139–162
Al-Taher F, Banaszewski K, Jackson L, Zweigenbaum J, Ryu D, Cappozzo J (2013) Rapid method for the determination of multiple mycotoxins in wines and beers by LC-MS/MS using a stable isotope dilution assay. J Agric Food Chem 61:2378–2384
Remiro R, Ibáñez-Vea M, González-Peñas E, Lizarraga E (2010) Validation of a liquid chromatography method for the simultaneous quantification of ochratoxin A and its analogues in red wines. J Chromatogr, A 1217:8249–8256
Meulenberg EP (2012) Immunochemical methods for ochratoxin A detection: a review. Toxins 4:244–266
Bazin I, Nabais E, Lopez-Ferber M (2010) Rapid visual tests: fast and reliable detection of ochratoxin A. Toxins 2:2230–2241. doi:10.3390/toxins2092230
Rusanova TY, Beloglazova NV, Goryacheva IY, Lobeau M, Van Peteghem C, De Saeger S (2009) Non-instrumental immunochemical tests for rapid ochratoxin A detection in red wine. Anal Chim Acta 653:97–102
Prieto-Simón B, Karube I, Saiki H (2012) Sensitive detection of ochratoxin A in wine and cereals using fluorescence-based immunosensing. Food Chem 135:1323–1329
Cho YJ, Lee DH, Kim DO, Min WK, Bong KT, Lee GG, Seo JH (2005) Production of a monoclonal antibody against ochratoxin A and its application to immunochromatographic assay. J Agric Food Chem 53:8447–8451
Wang XH, Liu T, Xu N, Zhang Y, Wang S (2007) Enzyme-linked immunosorbent assay and colloidal gold immunoassay for ochratoxin A: investigation of analytical conditions and sample matrix on assay performance. Anal Bioanal Chem 389:903–911
Lai W, Fung DYC, Xu Y, Liu R, Xiong Y (2009) Development of a colloidal gold strip for rapid detection of ochratoxin A with mimotope peptide. Food Control 20:791–795
Anfossi L, D’Arco G, Baggiani C, Giovannoli C, Giraudi G (2011) A lateral flow immunoassay for measuring ochratoxin A: development of a single system for maize, wheat and durum wheat. Food Control 22:1965–1970
Urusov AE, Kostenko SN, Sveshnikov PG, Zherdev AV, Dzantiev BB (2011) Immunochromatographic assay for the detection of ochratoxin A. J Anal Chem 66:770–776
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Farady Soc 11:55–75
Anfossi L, Calderara M, Baggiani C, Giovannoli C, Arletti E, Giraudi G (2010) Development and application of a quantitative lateral flow immunoassay for fumonisins in maize. Anal Chim Acta 682:104–109
Anfossi L, Baggiani C, Giovannoli C, Biagioli F, D'Arco G, Giraudi G (2013) Optimization of a lateral flow immunoassay for the ultrasensitive detection of aflatoxin M1 in milk. Anal Chim Acta 772:75–80
Byzova NA, Zvereva EA, Zherdev AV, Eremin SA, Sveshnikov PG, Dzantiev BB (2011) Pretreatment-free Immunochromatographic assay for the detection of streptomycin and its application to the control of milk and dairy products. Anal Chim Acta 701:209–217
Liu BH, Tsao ZJ, Wang JJ, Yu FY (2008) Development of a monoclonal antibody against ochratoxin A and its application in enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip. Anal Chem 80:7029–7035
Laycock MV, Donovan MA, Easy DJ (2010) Sensitivity of lateral flow tests to mixtures of saxitoxins and applications to shellfish and phytoplankton monitoring. Toxicon 55:597–605
Liu YH, Xie R, Guo YR, Zhu GN, Tang FB (2012) Comparison of homologous and heterologous formats in nanocolloidal gold-based immunoassays for parathion residue determination. J Environm Sci Health B 47:475–483
Horsiberger M, Rosset J (1977) Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem 25:295–305
Anfossi L, Baggiani C, Giovannoli C, Giraudi G (2009) Homogeneous immunoassay based on gold nanoparticles and visible absorption detection. Anal Bioanal Chem 394:507–512
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anfossi, L., Di Nardo, F., Giovannoli, C. et al. Increased sensitivity of lateral flow immunoassay for ochratoxin A through silver enhancement. Anal Bioanal Chem 405, 9859–9867 (2013). https://doi.org/10.1007/s00216-013-7428-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7428-6