Abstract
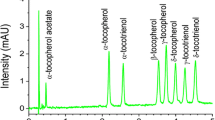
Reversed-phase liquid chromatographic (RPLC) separation of isomers and homologues of similar polarity is challenging. Tocopherol isomers and homologues are one such example. α, β, γ, and δ-tocopherols have been successfully separated by RPLC on triacontyl (C30) stationary phase. System suitability was tested by using four mobile phases, and observed chromatographic separations of β and γ-tocopherols were compared. Comparison indicated that methanol–tert-butyl methyl ether (TBME) 95:5 (v/v) at a flow rate of 0.75 mL min−1 was the best mobile phase. Detection systems were also evaluated on the basis of limit of quantification; it was concluded that fluorescence detection was best. The method was validated by analysis of two homologues and two isomers of tocopherol in sesame, maize, and soybean samples. MS coupled with an ESI interface in negative-ion mode [M − H]− was used for identification of individual components. It was concluded that addition of TBME to methanol was required to enhance the separation of β and γ-tocopherols, although methanol alone provided similar results. The applicability of the method to cereal, pulse, and oilseed samples was confirmed. The reproducibility of the procedure was good, with relative standard deviations in the range 1.7–3.9 %. Recovery of tocopherols added to sesame samples ranged from 91 to 99 %.

ᅟ





Similar content being viewed by others
References
Lee B, New A, Ong C (2003) Simultaneous determination of tocotrienols, tocopherols, retinol, and major carotenoids in human plasma. Clin Chem 49:2056–2066
Theriault A, Chao JT, Wang Q, Gapor A, Adeli K (1999) Tocotrienol: a review of its therapeutic potential. Clin Biochem 32:309–319
Yu W, Jia L, Park SK, Li J, Gopalan A, Simmons-Menchaca M, Sanders BG, Kline K (2009) Anticancer actions of natural and synthetic vitamin E forms: RRR-alpha-tocopherol blocks the anticancer actions of gamma-tocopherol. Mol Nutr Food Res 53:1573–1581
Hatam LJ, Kayden HJ (1979) A high-performance liquid chromatographic method for the determination of tocopherol in plasma and cellular elements of the blood. J Lipid Res 20:639–645
McMurray CH, Blanchflower WJ (1979) Determination of a-tocopherol in animal feedstuffs using high-performance liquid chromatography with spectrofluorescence detection. J Chromatogr 176:488–492
Ishibashi K, Abe K, Ohmae M, Katsui C (1977) Determination of tocopherols in red blood cells by high-speed chromatography. Vitamins (Japan) 51:415–422
Rani A, Kumar V, Verma SK, Shakya AK, Chauhan GS (2007) Tocopherol content and profile of soybean: genotypic variability and correlation studies. J Am Oil Chem Soc 84:377–383
Amaral JS, Casal S, Torres D, Seabra RM, Oliveira BPP (2005) Simultaneous determination of tocopherols and tocotrienols in hazelnuts by normal phase liquid chromatography. Anal Sci 21:1545–1548
Warner K, Mounts TL (1990) Analysis of tocopherols and phytosterols in vegetable oils by hplc with evaporative light-scattering detection. J Am Oil Chem Soc 67:827–831
Lehman J, Martin HL (1982) Improved direct determination of alpha- and gamma-tocopherols in plasma and platelets by liquid chromatography, with fluorescence detection. Clin Chem 28:1784–1787
Panfili G, Fratianni A, Irano M (2003) Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J Agric Food Chem 51:3940–3944
Barros L, Correia DM, Ferreira ICFR, Baptista P, Santos-Buelga C (2008) Optimization of the determination of tocopherols in Agaricus sp. edible mushrooms by a normal phase liquid chromatographic method. Food Chem 110:1046–1050
Chauveau-Duriot B, Doreau M, Noziere P, Graulet B (2010) Simultaneous quantification of carotenoids, retinol, and tocopherols in forages, bovine plasma, and milk: validation of a novel UPLC method. Anal Bioanal Chem 397:777–790
Abidi SL (1999) Reversed-phase retention characteristics of tocotrienol antioxidants. J Chromatogr A 844:67–75
Sander LC, Sharpless KE, Craft NE, Wise SA (1994) Development of engineered stationary phases for the separation of carotenoid isomers. Anal Chem 66:1667–1674
Pursch M, Strohschein S, Handel H, Albert K (1996) Temperature dependent behavior of C30 interphases. A solid-state NMR and LC-NMR study. Anal Chem 68:386–393
Rimmer CA, Sander LC, Wise SA (2005) Selectivity of long chain stationary phases in reversed-phase liquid chromatography. Anal Bioanal Chem 382:698–707
Sharpless KE, Thomas JB, Sander LC, Wise SA (1996) Liquid chromatographic determination of carotenoids in human serum using an engineered C30 and C18 stationary phase. J Chromatogr B 678:187–195
Britz SJ, Prasad PVV, Moreau RA, Allen LH Jr, Kremer DF, Boote KJ (2007) Influence of growth temperature on the amounts of tocopherols, tocotrienols, and γ-oryzanol in brown rice. J Agric Food Chem 55:7559–7565
Snyder LR, Glajch JL, Kirkland JJ (1997) Practical HPLC Method Development, 2nd edn. Wiley, New York
Manzi P, Panfili G, Pizzoferrato L (1996) Normal and reversed-phase HPLC for more complete evaluation of tocopherols, retinols, carotenes and sterols in dairy products. Chromatographia 43:89–93
Chotimarkorn C, Benjakul S, Silalai N (2008) Antioxidant components and properties of five long-grained rice bran extracts from commercial available cultivars in Thailand. Food Chem 111:636–641
Fallas MM, Tanaka N, Buckenmaeir SMC, McCalley DV (2013) Influence of phase type and solute structure on changes in retention with pressure in reversed-phase high performance liquid chromatography. J Chromatogr A 1297:37–45
Gritti F, Martin M, Guiochon G (2005) Influence of pressure on the properties of chromatographic columns. II. The column hold up volume. J Chromatogr A 1070:13–22
Gritti F, Guiochon G (2005) Influence of the pressure on the properties of chromatographic columns: III. Retention volume of thiourea, hold-up volume, and compressibility of the C18-bonded layer. J Chromatogr A 1075:117–126
Puspitasari-Nienaber NL, Ferruzzi MG, Schwartz SJ (2002) Simultaneous detection of tocopherols, carotenoids, and chlorophylls in vegetable oils by direct injection C30 RP-HPLC with coulometric electrochemical array detection. J Am Oil Chem Soc 79:633–640
Balz M, Shulte E, Their HP (1992) HPLC Separation of Tocopherols and Tocotrienols. Fat Sci Technol 94:209–213
Sander LC, Pursch M, Wise SA (1999) Shape selectivity for constrained solutes in reversed-phase liquid chromatography. Anal Chem 71:4821–4830
Cunha SC, Amaral JS, Fernandes JO, Oliveira MBPP (2006) Quantification of tocopherols and tocotrienols in portuguese olive oils using HPLC with three different detection systems. J Agric Food Chem 54:3351–3356
Rodrigo N, Alegra A, Barber R, Farr R (2002) High performance liquid chromatographic determination of tocopherols in infant formulas. J Chromatogr A 947:97–102
Bustamante-Rangel M, Delgado-Zamarreno MM, Sanchez-Perez A, Carabias-Martınez R (2007) Determination of tocopherols and tocotrienols in cereals by pressurized liquid extraction–liquid chromatography–mass spectrometry. Anal Chim Acta 587:216–221
Lanina SA, Toledo P, Sampels S, Kamal-Eldin A, Jastrebova JA (2007) Comparison of reversed-phase liquid chromatography-mass spectrometry with electrospray and atmospheric pressure chemical ionization for analysis of dietary tocopherols. J Chromatogr A 1157:159–170
Akasaka K, Ohrui H (2004) Chiral discrimination of branched chain fatty acids by reverse phase HPLC after labeling with a chiral fluorescent conversion agent. Biosci Biotechnol Biochem 68:153–184
Kamal-Ekdin A, Gorgen S, Pettersson J, Lampi A (2000) Normal-phase high-performance liquid chromatography of tocopherols and tocotrienols comparison of different chromatographic columns. J Chromatogr A 881:217–227
Preedy VR, Watson RR (2007) The encyclopedia of vitamin E. CABI publishing. 2007, 1
Acknowledgments
The authors acknowledge the support provided by the Head, Division of Agricultural Chemicals, Indian Agricultural Research Institute, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saha, S., Walia, S., Kundu, A. et al. Effect of mobile phase on resolution of the isomers and homologues of tocopherols on a triacontyl stationary phase. Anal Bioanal Chem 405, 9285–9295 (2013). https://doi.org/10.1007/s00216-013-7336-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7336-9