Abstract
Mycotoxins are toxic fungal secondary metabolites that frequently contaminate food and feed worldwide, and hence represent a major hazard for food and feed safety. To estimate human exposure arising from contaminated food, so-called biomarker approaches have been developed as a complementary biomonitoring tool besides traditional food analysis. The first methods based on radioimmunoassays and enzyme-linked immunosorbent assays as well as on liquid chromatography were developed in the late 1980s and early 1990s for the carcinogenic aflatoxins and in the last two decades further tailor-made methods for some major mycotoxins have been published. Since 2010, there has been a clear trend towards the development and application of multianalyte methods based on liquid chromatography–electrospray ionization tandem mass spectrometry for assessment of mycotoxin exposure made possible by the increased sensitivity and selectivity of modern mass spectrometry instrumentation and sophisticated sample cleanup approaches. With use of these advanced methods, traces of mycotoxins and relevant breakdown and conjugation products can be quantified simultaneously in human urine as so-called biomarkers and can be used to precisely describe the real exposure, toxicokinetics, and bioavailability of the toxins present. In this article, a short overview and comparison of published multibiomarker methods focusing on the determination of mycotoxins and relevant excretion products in human urine is presented. Special attention is paid to the main challenges when analyzing these toxic food contaminants in urine, i.e., very low analyte concentrations, appropriate sample preparation, matrix effects, and a lack of authentic, NMR-confirmed calibrants and reference materials. Finally, the progress in human exposure assessment studies facilitated by these analytical methods is described and an outlook on probable developments and possibilities is presented.

Mycotoxin exposure assessment: traditional food analysis compared to the innovative, complementary biomarker approach
Similar content being viewed by others
Introduction
Toxic fungal secondary metabolites, so-called mycotoxins, are a global hazard for food safety by frequently contaminating food and feed. To estimate the risk of exposed populations, traditional exposure assessment comprises the analysis of foodstuff and evaluation of dietary recalls or the estimation of average consumption patterns. To overcome the disadvantages of this indirect approach, such as a lack of information on individual exposure, toxicokinetics, and bioavailability, biomarker approaches were developed as a biomonitoring tool for some major mycotoxins (Fig. 1). Baldwin et al. [1] reviewed biomarker research for the commercially most important mycotoxins and defined biomarkers as measurable biochemical or molecular indicators of either exposure (exposure biomarker) or biological response (effect biomarker) to a mycotoxin that can be specifically linked to the proximate cause. Typical biomarkers of exposure are the parent toxins themselves, protein or DNA adducts, and/or major phase I or phase II metabolites (e.g. glucuronide conjugates), which are measured in biological fluids such as urine or plasma/serum, and are related to the actual intake of the toxin through contaminated food. In an excellent review, the role of biomarkers in the evaluation of human health concerns caused by mycotoxins was published recently. Here a biomarker of exposure was defined as a biological measure which is correlated with the quantity of the xenobiotic ingested, resulting in improved exposure classification over more traditional approaches [2]. It was highlighted that validation of such a biomarker requires demonstration of (a) assay robustness, (b) intake versus biomarker level, and (c) stability of stored samples.
Biomarker research for human exposure assessment entered the mycotoxin research arena in the late 1980s and early 1990s when extensive studies on the carcinogenic aflatoxins were conducted [3–5]. They have been essential for the establishment of the etiologic role of aflatoxins in human disease through better estimates of exposure, expanded knowledge of the mechanisms of disease pathogenesis, and as tools for implementing and evaluating preventive interventions [5]. Three aflatoxin biomarkers were validated by the establishment of a dose–response relationship: in urine the level of the hydroxylated metabolite aflatoxin M1 (AFM1) was between 1.2 and 2.2 % of that of ingested aflatoxin B1 (AFB1) [6], while the level of the aflatoxin–N 7-guanine adduct ranged from 0.05 to 3.25 μg/L, with approximately 0.2 % of ingested AFB1 excreted during a 3-day period [7]. AFM1 was analyzed by a competitive direct enzyme-linked immunosorbent assay (ELISA) whereas aflatoxin–N 7-guanine was measured by high-performance liquid chromatography (HPLC) following elution from an antibody affinity column. In serum the aflatoxin–lysine adduct can be obtained through digestion of the aflatoxin–albumin adduct [8]. Later in the 1990s work on ochratoxin A (OTA) [9] and the fumonisins [10] was conducted mainly based on HPLC with fluorescence detection. However, occasionally radioimmunoassays, ELISA, and liquid chromatography–tandem mass spectrometry (LC-MS/MS) have been applied as well. Excretion of fumonisin B1 (FB1) in urine was recently estimated to be on average 0.075 % of the FB1 intake in South African women (n = 22) [11], whereas the estimates were significantly higher (0.5 %) in a US study (n = 8) [12]. Despite this very low excretion rate and issues associated with interindividual variability and rapid clearance, urinary FB1 was recommended as a valuable biomarker for fumonisin exposure and risk assessment. Most fumonisin biomarker research conducted within the last two decades was related to the inhibition of the sphinganine N-acetyltransferase (ceramide synthase) and subsequent sphingolipid biosynthesis disruption initiated by fumonisins. A correlation between fumonisin intake and the sphinganine-to-sphingosine ratio or an elevated sphinganine level was found to be useful in animals but not in humans and constitutes a typical biomarker of effect [10]. The first biomarker research on the trichothecene deoxynivalenol (DON, vomitoxin) was initiated in 2003 when Meky et al. [13] developed an LC-MS-based assay to measure the sum of free DON and DON glucuronides (DON-GlcAs) combined after enzymatic hydrolysis and use of an immunoaffinity column (IAC) as a sum parameter in human and rat urine. Further LC-MS/MS methods were developed for the determination of DON and DON-GlcA using either a synthetically produced authentic reference standard [14] or the hypothetical mass [15] for the detection of the glucuronide(s). A major limitation of proper exposure assessment including ideally all relevant mycotoxins and their biotransformation products was the lack of sufficient sensitivity and selectivity.
As a result of the advent of the latest generation of high-performance LC-MS/MS instruments, a clear trend towards the development and application of multianalyte methods in mycotoxin biomarker research can be observed. Purification of the analytes is often achieved by using sophisticated sample cleanup approaches with subsequent separation by liquid chromatography and detection using triple-quadrupole analyzers coupled via an electrospray ionization (ESI) interface. However, the latest studies have also successfully applied the so-called dilute and shoot approach by omitting any cleanup step [16]. This article provides a short overview and comparison of published multibiomarker methods, discusses challenges associated with very low analyte concentrations, sample preparation, matrix effects, and a lack of calibrants and certified reference materials, and describes the progress in human exposure assessment studies facilitated by these methods.
LC-MS/MS-based multibiomarker methods
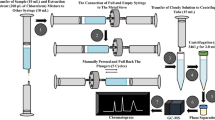
The first method described for the determination of various mycotoxin biomarkers in human urine was developed by Ahn et al. [17]. To achieve sufficient sensitivity and selectivity, AFM1, OTA, FB1, and fumonisin B2 were concentrated using three separate IACs. The eluates were pooled, dried under a stream of nitrogen, and resolved in a mixture of acetonitrile and water . Also two other published multibiomarker methods used the selectivity of antibodies by applying a novel multi-IAC column (Myco6in1™, Vicam) which comprises antibodies specific for aflatoxins, OTA, fumonisins, DON, zearalenone (ZEN), T-2 toxin, and HT-2 toxin [18, 19]. The first method did not include AFM1, but instead included the aflatoxins B1, B2, G1 and G2, for which no correlation with food intake had been achieved in the past [2]. In addition, no enzymatic hydrolysis was performed despite the extensive glucuronidation of DON [13] and ZEN [20] one can expect in such studies. In constrast, the method of Solfrizzo et al. [19] used β-glucuronidase-assisted hydrolysis, resulting in increased levels of the parent toxins. Besides the IAC enrichment, a second step of sample preparation using solid-phase extraction (SPE; Oasis HLB, Waters) was conducted to overcome issues associated with low DON and deepoxy-DON recoveries. The advanced cleanup procedure resulted in lower limits of detection (LODs) of this method compared with that of Rubert et al. [18] although a less sensitive mass spectrometer was used (Table 1). Our group chose a time- and cost-effective “dilute and shoot” approach for sample preparation, where the urine sample is simply diluted 1:10 with acetonitrile/water (10:90) and injected directly into the LC-MS/MS system, to facilitate the quantification of 15 analytes [16]. A chromatogram of a blank urine sample spiked with reference standards is illustrated in Fig. 2. Besides the simplification, the advantage of this workflow is the full recovery of the polar conjugates such as glucuronides which are frequently lost during sample cleanup [21]. By implementation of these key excretion metabolites in a method using authentic reference standards, it is possible to investigate the metabolism of a certain mycotoxin as successfully exemplified for DON in vitro [22] and in vivo [23, 24]. The disadvantage of the dilute and shoot approach is the prerequisite of the latest state-of-the art triple-quadrupole mass analyzer to achieve the very low LODs required. Even when these highly advanced instruments are used, it is moderate to high exposure rather than very low background traces that is detectable. A method developed by Njumbe Ediage et al. [25] covers seven mycotoxins and several important conjugation and breakdown products (in total 18 analytes). Sample cleanup was optimized in a progressive procedure where urine samples were extracted with ethyl acetate/formic acid (99:1, v/v) followed by strong anion exchange (SAX) SPE cleanup of the acidified aqueous fraction. The combined extracts of the evaporated organic phase and the SAX eluate were injected into the LC-MS/MS system. Owing to the high concentration factor, the reported recovery was between approximately 45 and 100 %. In contrast to results obtained by various groups [15, 23, 26, 27], no DON-GlcA was detected in urine samples naturally contaminated with DON. This might indicate a loss of those conjugates during cleanup despite successful validation. However, this could also be because DON-3-GlcA was analyzed exclusively rather than DON-15-GlcA which was recently suggested as the human main excretion product [23]. The analytes included and the performance characteristics of the five multibiomarker methods described above are compared in Table 1. For quantitative analysis of urine samples, all methods were performed in selected reaction monitoring (SRM) mode. Methods 3 and 4 recently showed good agreement for most of the investigated analytes in a mini interlaboratory comparison [28]. Although in all the methods developed urine was the matrix of choice, there are limitations related to this approach, e.g., differing urine excretion owing to different fluid intakes. This can be overcome partially by normalization for the creatinine concentration of a urine sample. In exposure studies it is recommended to collect 24-h urine instead of first morning or spot urine samples if possible as spot samples are usually not representative of the excretion throughout a day [24]. In addition, urinary excretion mainly represents recent mycotoxin intake, whereas measurements in plasma/serum are more likely to represent long-term exposure.
Chromatogram from selected reaction monitoring (SRM) of a blank urine sample spiked with reference standards. Between 5 and 10 min, the analytes were monitored in negative ionization mode only (period I), whereas between 10 and 15 min both polarity modes were measured simultaneously using fast polarity switching (period II). AFM 1 aflatoxin M1, FB 1 fumonisin B1, FB 2 fumonisin B2, DON deoxynivalenol, DOM de-epoxy deoxynivalenol, GlcA glucuronide, NIV nivalenol, OTA ochratoxin A, ZEL zearalenol, ZEN zearalenone. (Adapted from [16])
Analytical challenges
Sample preparation
A major challenge in mycotoxin biomarker research are the extremely low analyte concentrations present in biological fluids following dietary exposure. Hence, appropriate sample preparation protocols are crucial to obtain acceptable LODs. This is, however, hampered by the great chemical diversity of analytes typically included in multibiomarker methods. This issue becomes even more complex once polar conjugates such as glucuronides are included as they are frequently lost during common cleanup approaches such as SPE or IAC procedures [16, 21]. The five methods presented in the previous section and in Table 1 illustrate different concepts in an excellent way. The great advantage of the methods using IAC cleanup is the specific retention of the target compounds only. Thereby, high enrichment factors are obtained without concentrating also potentially interfering matrix compounds as they are removed efficiently. The major disadvantage is the preselection of analytes by the column chosen depending on the antibodies used. Therefore, usually no conjugates or other biomarkers/analytes of interest can be included in a method. Furthermore, enzymatic hydrolysis should be performed to include conjugates, and the overall procedure is time-consuming and costly and requires a labor-intensive sample preparation. This is in contrast to the dilute and shoot approach, where a urine sample is centrifuged, diluted, and analyzed without further pretreatment. However, to overcome matrix effects and interfering matrix peaks, eluents, the chromatographic gradient, and the dilution factor need to be carefully optimized [14, 16]. Njumbe Ediage et al. [25] investigated different procedures including dilute and shoot, dilute, evaporate, and shoot, liquid–liquid extraction, and two different SPE cartridges (SAX and Oasis HLB). They concluded that the LODs obtained with SAX columns were threefold to ninefold lower compared with those obtained with Oasis HLB columns, whereas the approaches based on sample dilution yielded unfeasibly high LODs and significant signal enhancement for ZEN and FB1. Various SPE cartridges (Oasis HLB and MAX, Sigma Supel-Select HLB, Sequant ZIC-HILIC, Bakerbond Polar Plus) have also been tested during method development of the established dilute and shoot method but failed to retain the polar glucuronide conjugates, with the exception of the Oasis HLB [16] and the ZIC-HILIC cartridges when using optimized protocols.
Matrix effects and peaks
Co-eluting matrix components can negatively influence the accuracy of quantitative methods through ion suppression or enhancement in the ion source. This is particularly true for ESI, where the competition for electrical charges or the effect on the evaporation of ESI droplets can lead to significant ion suppression [29]. Hence, it is of great importance to thoroughly investigate these effects during method development and validation. Ion suppression can be reduced efficiently by careful optimization of the eluents and gradient. However, this is not trivial and is a particular issue in multianalyte methods, where compromises are unavoidable. Matrix effects can be controlled by using matrix-matched calibration [19], inclusion of internal standards [17, 30], or correction of results with the apparent recovery [16]. However, when matrix-matched calibration or apparent recovery for the correction of results is used, it still needs to be considered that urine samples can differ in their concentration, thereby influencing matrix effects. This depends largely on the volume of drinks consumed by an individual prior to sample donation. Therefore, the blank urine which is used for preparation of matrix-matched standards or the spiked samples, respectively, needs to be chosen with the greatest care and the effect of differing urine sample concentrations should be investigated during validation.
Another major issue is the frequent co-elution of matrix compounds. This requires careful selection of SRM transitions in order to minimize background noise as well as interfering peaks that might trigger false-positive results. Descriptive examples are illustrated for an AFM1 interference by Ahn et al. [17] and for zearalenone-14-glucuronide (ZEN-14-GlcA) in Fig. 3. During common tandem mass spectrometric compound optimization, usually the two most abundant fragment ions are chosen as quantifier and qualifier ions, respectively. However, in challenging biomarker applications, one should consider several SRM candidates in order to select specific fragment ions. This evaluation must include the injection of spiked matrix samples to identify potential interferences and is particularly required if no proper sample cleanup was performed. This issue is visualized in Fig. 3.
SRM chromatogram of a blank urine sample spiked at a level of 12.5 μg/L ZEN-14-GlcA. It is obvious that the transition m/z 493 [M-H]-→131 results in a far better signal-to-noise ratio than the other product ions despite its lower absolute abundance. Hence, this fragment should be chosen as a quantifier ion. In addition, the more intense transitions comprise many interferences which mimic ZEN-14-GlcA and thus potentially may lead to false-positive results
Lack of authentic reference standards and certified reference materials
In the past, most biomarker methods focused on parent mycotoxins rather than on conjugated forms as no (certified) calibrants are commercially available for these metabolites. Despite this caveat, considerable progress has been achieved in the direct quantification of mycotoxin conjugates without the need for enzymatic hydrolysis. By application of this direct approach, problems such as the loss of information on the analyte’s structure and its detoxification potential, but also incomplete hydrolysis and the time-consuming sample preparation can be overcome. Glucuronide conjugates have been synthesized either using chemical synthesis as in the case of DON-3-GlcA [31] and ZEN-14-GlcA [32] or by in vitro assays using liver microsomes. With use of this approach, GlcAs of DON [27, 33], ZEN and metabolites [34], and T-2 toxin and HT-2 toxin [35] were obtained in small quantities. An important quality control measure is the use of certified reference materials including well-characterized calibrants to monitor the performance of a certain laboratory. However, for mycotoxin biomarkers, i.e., mycotoxins and their conjugates, there is no matrix reference material available that would make it possible to assess the measurement performance in the analysis of biologically important matrices such as human or animal urine, plasma/serum, or feces. This is critical especially in view of the complex biological matrices and makes efforts such as a recent interlaboratory comparison [28] even more important to ensure analytical accuracy. The preliminary results obtained in this study which determined up to eight mycotoxin biomarkers in human urine showed good agreement between most analytes. The overall rate of satisfactory z scores [36] (|z| ≤ 2) was 85 % (68 of 80 results), with unsatisfactory or questionable z scores obtained for FB1, OTA, and α-zearalenol.
Application of LC-MS/MS methods in exposure studies
The multibiomarker methods presented have been applied in several pilot studies to prove their applicability and to estimate mycotoxin exposure in the populations/individuals tested. In general, the application of these methods resulted in advanced data on exposure patterns and revealed new findings on co-exposure to the mycotoxin combinations reported in Table 2. This is a significant advancement compared with the results presented in the only reported co-exposure study in which three separate methods based on ELISA, HPLC with fluorescence detection, and LC-MS/MS were applied to reveal exposure to aflatoxin and DON in pregnant women from Egypt [37]. An example of the relevance of the reported new exposure data is the extent of co-exposure observed in samples from Cameroonian individuals [16]. Overall, in 110 samples (63 %, n = 175) at least one analyte was detected, with a maximum of six analytes (AFM1, FB1, OTA, DON, DON-15-GlcA, nivalenol) detected in a single individual simultaneously, a severe co-exposure that had never been reported before (see also Table 2). In this study additionally the first quantification of ZEN-14-GlcA and nivalenol in naturally contaminated human urine was described. In a very recent South African survey among women living in a rural, high esophageal cancer region, two different multibiomarker methods and, in addition, two single-target LC-MS/MS methods were used and indicated frequent mycotoxin co-exposure for the first time in South Africa. Furthermore, the first finding of urinary DON, ZEN, their conjugates, and OTA in this region and an advanced understanding of toxicokinetic patterns by direct determination of conjugation and hydroxylation products of DON and ZEA was achieved [38]. In an Austrian pilot survey, the structure of DON-15-GlcA was tentatively elucidated and identified as the major conjugation product in human urine. Furthermore, it was estimated that a significant number of study participants exceeded the tolerable daily intake established for DON [23]
Outlook
The current trend of multianalyte methods in mycotoxin biomarker research will certainly continue. We expect these methods to be optimized and validated for even more challenging matrices such as feces and plasma as done for single-target methods in the past [2]. The methods developed will significantly contribute to improved exposure assessment. Thereby, they offer a new innovative and complementary way of quantifying the risks associated with mycotoxins, and will be of increasing importance besides traditional food analysis.
Driven by the increasing sensitivity of modern mass spectrometers, more detailed in vivo toxicokinetic studies will be performed directly in humans following low toxin intake via naturally contaminated food. These experiments have mainly been restricted to animals in the past because of high doses. Thereby, metabolism and detoxification routes will be discovered as recently demonstrated for DON and ZEN [24] to support advanced risk assessment. Furthermore, it is expected that more biomarkers of mycotoxin exposure will be validated using these methods by means of a dose–response relationship.
We also expect more laboratories to be involved in efforts to synthesize novel mycotoxin conjugates such as α-zearalenol glucuronide, β-zearalenol glucuronide, OTA glucuronide, and ochratoxin α glucuronide as calibrants and implement them in multianalyte methods. This includes regulated toxins but also mycotoxins which have rarely or not been addressed yet by biomarker research, such as T-2/HT-2 toxin, nivalenol, citrinin, Alternaria toxins, and moniliformin. The quest for new key metabolites will be supported by high-resolution mass spectrometry and increasingly sensitive triple-quadrupole analyzers.
Ultimately, the multibiomarker approach could serve in the identification of what are some of the most important mycotoxin mysteries: the role of mycotoxins in chronic disease caused by low-dose long-term background exposure through the intake of contaminated food and the toxicological risks posed by combinations of mycotoxins of frequent natural occurrence.
References
Baldwin TT, Riley RT, Zitomer NC, Voss KA, Coulombe RA Jr, Pestka JJ, Williams DE, Glenn AE (2011) The current state of mycotoxin biomarker development in humans and animals and the potential for application to plant systems. World Mycotoxin J 4:257–270
Turner PC, Flannery B, Isitt C, Ali M, Pestka J (2012) The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr Res Rev 25:162–179
Leong YH, Latiff AA, Ahmad NI, Rosma A (2012) Exposure measurement of aflatoxins and aflatoxin metabolites in human body fluids. A short review. Mycotoxin Res 28:79–87
Wild CP, Turner PC (2002) The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 17:471–481
Kensler TW, Roebuck BD, Wogan GN, Groopman JD (2011) Aflatoxin: a 50-year odyssey of mechanistic and translational toxicology. Toxicol Sci 120:28–48
Zhu JQ, Zhang LS, Hu X (1987) Correlation of dietary aflatoxin B1 levels with excretion of aflatoxin M1 in human urine. Cancer Res 47:1848–1852
Groopman JD, Kensler TW (1993) Molecular biomarkers for human chemical carcinogen exposures. Chem Res Toxicol 6:764–770
Walton M, Egner P, Scholl PF, Walker J, Kensler TW, Groopman JD (2001) Liquid chromatography electrospray-mass spectrometry of urinary aflatoxin biomarkers: characterization and application to dosimetry and chemoprevention in rats. Chem Res Toxicol 14:919–926
Scott PM (2005) Biomarkers of human exposure to ochratoxin A. Food Addit Contam 22:99–107
Shephard GS, van der Westhuizen L, Sewram V (2007) Biomarkers of exposure to fumonisin mycotoxins: a review. Food Addit Contam 24:1196–1201
Van Der Westhuizen L, Shephard GS, Burger HM, Rheeder JP, Gelderblom WCA, Wild CP, Gong YY (2011) Fumonisin B1 as a urinary biomarker of exposure in a maize intervention study among South African subsistence farmers. Cancer Epidemiol Biomarkers Prev 20:483–489
Riley RT, Torres O, Showker JL, Zitomer NC, Matute J, Voss KA, Gelineau-van Waes J, Maddox JR, Gregory SG, Ashley-Koch AE (2012) The kinetics of urinary fumonisin B1 excretion in humans consuming maize-based diets. Mol Nutr Food Res 56:1445–1455
Meky FA, Turner PC, Ashcroft AE, Miller JD, Qiao YL, Roth MJ, Wild CP (2003) Development of a urinary biomarker of human exposure to deoxynivalenol. Food Chem Toxicol 41:265–273
Warth B, Sulyok M, Berthiller F, Schuhmacher R, Fruhmann P, Hametner C, Adam G, Fröhlich J, Krska R (2011) Direct quantification of deoxynivalenol glucuronide in human urine as biomarker of exposure to the Fusarium mycotoxin deoxynivalenol. Anal Bioanal Chem 401:195–200
Lattanzio VMT, Solfrizzo M, De Girolamo A, Chulze SN, Torres AM, Visconti A (2011) LC-MS/MS characterization of the urinary excretion profile of the mycotoxin deoxynivalenol in human and rat. J Chromatogr B Anal Technol Biomed Life Sci 879:707–715
Warth B, Sulyok M, Fruhmann P, Mikula H, Berthiller F, Schuhmacher R, Hametner C, Abia WA, Adam G, Fröhlich J, Krska R (2012) Development and validation of a rapid multi-biomarker liquid chromatography/tandem mass spectrometry method to assess human exposure to mycotoxins. Rapid Commun Mass Spectrom 26:1533–1540
Ahn J, Kim D, Kim H, Jahng KY (2010) Quantitative determination of mycotoxins in urine by LC-MS/MS. Food Addit Contam Part A 27:1674–1682
Rubert J, Soriano JM, Mañes J, Soler C (2011) Rapid mycotoxin analysis in human urine: a pilot study. Food Chem Toxicol 49:2299–2304
Solfrizzo M, Gambacorta L, Lattanzio VMT, Powers S, Visconti A (2011) Simultaneous LC-MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, α and β-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal Bioanal Chem 401:2831–2841
Mirocha CJ, Pathre SV, Robison TS (1981) Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet Toxicol 19:25–30
Veršilovskis A, Huybrecht B, Tangni EK, Pussemier L, De Saeger S, Callebaut A (2011) Cross-reactivity of some commercially available deoxynivalenol (DON) and zearalenone (ZEN) immunoaffinity columns to DON- and ZEN-conjugated forms and metabolites. Food Addit Contam Part A 28:1687–1693
Maul R, Warth B, Kant J-S, Schebb NH, Krska R, Koch M, Sulyok M (2012) Investigation of the hepatic glucuronidation pattern of the Fusarium mycotoxin deoxynivalenol in various species. Chem Res Toxicol 25:2715–2717
Warth B, Sulyok M, Fruhmann P, Berthiller F, Schuhmacher R, Hametner C, Adam G, Fröhlich J, Krska R (2012) Assessment of human deoxynivalenol exposure using an LC-MS/MS based biomarker method. Toxicol Lett 211:85–90
Warth B, Sulyok M, Berthiller F, Schuhmacher R, Krska R (2013) New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol Lett 220(1):88–94
Njumbe Ediage E, Diana Di Mavungu J, Song S, Wu A, Van Peteghem C, De Saeger S (2012) A direct assessment of mycotoxin biomarkers in human urine samples by liquid chromatography tandem mass spectrometry. Anal Chim Acta 741:58–69
Turner PC, Hopton RP, White KL, Fisher J, Cade JE, Wild CP (2011) Assessment of deoxynivalenol metabolite profiles in UK adults. Food Chem Toxicol 49:132–135
Wu X, Murphy P, Cunnick J, Hendrich S (2007) Synthesis and characterization of deoxynivalenol glucuronide: its comparative immunotoxicity with deoxynivalenol. Food Chem Toxicol 45:1846–1855
Solfrizzo M, Gambacorta L, Warth B, White K, Srey C, Sulyok M, Krska R, Gong YY (2013) Comparison of single and multi-analyte methods based on LC-MS/MS for mycotoxin biomarker determination in human urine. World Mycotoxin J. doi:10.3920/WMJ2013.1575
Schuhmacher R, Sulyok M, Krska R (2008) Recent developments in the application of liquid chromatography–tandem mass spectrometry for the determination of organic residues and contaminants. Anal Bioanal Chem 390:253–256
Turner PC, Burley VJ, Rothwell JA, White KL, Cade JE, Wild CP (2008) Dietary wheat reduction decreases the level of urinary deoxynivalenol in UK adults. J Expo Sci Environ Epidemiol 18:392–399
Fruhmann P, Warth B, Hametner C, Berthiller F, Horkel E, Adam G, Sulyok M, Krska R, Fröhlich J (2012) Synthesis of deoxynivalenol-3-ß-D-O-glucuronide for its use as biomarker for dietary deoxynivalenol exposure. World Mycotoxin J 5:127–132
Mikula H, Hametner C, Berthiller F, Warth B, Krska R, Adam G, Fröhlich J (2012) Fast and reproducible chemical synthesis of zearalenone-14-β, D-glucuronide. World Mycotoxin J 5:289–296
Uhlig S, Ivanova L, Fæste CK (2013) Enzyme-assisted synthesis and structural characterization of the 3-, 8-, and 15-glucuronides of deoxynivalenol. J Agric Food Chem 61:2006–2012
Stevenson DE, Hansen RP, Loader JI, Jensen DJ, Cooney JM, Wilkins AL, Miles CO (2008) Preparative enzymatic synthesis of glucuronides of zearalenone and five of its metabolites. J Agric Food Chem 56:4032–4038
Welsch T, Humpf H-U (2012) HT-2 toxin 4-glucuronide as new T-2 Toxin metabolite: enzymatic synthesis, analysis, and species specific formation of T-2 and HT-2 toxin glucuronides by rat, mouse, pig, and human liver microsomes. J Agric Food Chem 60:10170–10178
Thompson M, Ellison SLR, Wood R (2006) The international harmonized protocol for the proficiency testing of analytical chemistry laboratories. Pure Appl Chem 78:145–196
Piekkola S, Turner PC, Abdel-Hamid M, Ezzat S, El-Daly M, El-Kafrawy S, Savchenko E, Poussa T, Woo JCS, Mykkänen H, El-Nezami H (2012) Characterisation of aflatoxin and deoxynivalenol exposure among pregnant Egyptian women. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 29:962–971
Shephard GS, Burger H-M, Gambacorta L, Gong Y, Krska R, Rheeder JP, Solfrizzo M, Srey C, Sulyok M, Visconti A, Warth B, Van der Westhuizen L (2012) Urinary biomarkers of multiple mycotoxin exposure in rural subsistence farmers in former Transkei, South Africa. In: Book of abstracts MycoRed North America, June 24–28 (2012). Carleton University, Ottawa
Acknowledgments
The authors acknowledge the support of the EC (KBBE-2007-22269-2 MYCORED) and the graduate school program Applied Bioscience Technology (AB-Tec) of Vienna University of Technology in cooperation with the University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Warth, B., Sulyok, M. & Krska, R. LC-MS/MS-based multibiomarker approaches for the assessment of human exposure to mycotoxins. Anal Bioanal Chem 405, 5687–5695 (2013). https://doi.org/10.1007/s00216-013-7011-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7011-1