Abstract
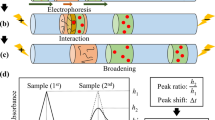
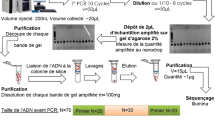
The recognition of targets such as biomacromolecules, viruses and cells by their aptamers is crucial in aptamer-based biosensor platforms and research into protein function. However, it is difficult to evaluate the binding constant of aptamers and their targets that are hard to purify and quantify, especially when the targets are undefined. Therefore, we aimed to develop a modified capillary electrophoresis based method to determine the dissociation constant of aptamers whose targets are hard to quantify. A protein target, human thrombin, and one of its aptamers were used to validate our modified method. We demonstrated that the result calculated by our method, only depending on the aptamer’s concentrations, was consistent with the classical method, which depended on the concentrations of both the aptamers and the targets. Furthermore, a series of DNA aptamers binding with avian influenza virus H9N2 were confirmed by a four-round selection of capillary electrophoresis–systematic evolution of ligands by exponential enrichment, and we identified the binding constant of these aptamers by directly using the whole virus as the target with the modified method. In conclusion, our modified method was validated to study the interaction between the aptamer and its target, and it may also advance the evaluation of other receptor–ligand interactions.



Similar content being viewed by others
References
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Fischer NO, Tarasow TM, Tok JB (2007) Aptasensors for biosecurity applications. Curr Opin Chem Biol 11:316–328
Torres-Chavolla E, Alocilja EC (2009) Aptasensors for detection of microbial and viral pathogens. Biosens Bioelectron 24:3175–3182
Iliuk AB, Hu L, Tao WA (2011) Aptamer in bioanalytical applications. Anal Chem 83:4440–4452
Tahiri-Alaoui A, Frigotto L, Manville N, Ibrahim J, Romby P, James W (2002) High affinity nucleic acid aptamers for streptavidin incorporated into bi-specific capture ligands. Nucleic Acids Res 30:e45
O’Brien KB, Esguerra M, Miller RF, Bowser MT (2004) Monitoring neurotransmitter release from isolated retinas using online microdialysis-capillary electrophoresis. Anal Chem 76:5069–5074
Wang Z, Wilkop T, Xu D, Dong Y, Ma G, Cheng Q (2007) Surface plasmon resonance imaging for affinity analysis of aptamer-protein interactions with PDMS microfluidic chips. Anal Bioanal Chem 389:819–825
Golub E, Pelossof G, Freeman R, Zhang H, Willner I (2009) Electrochemical, photoelectrochemical, and surface plasmon resonance detection of cocaine using supramolecular aptamer complexes and metallic or semiconductor nanoparticles. Anal Chem 81:9291–9298
Jing M, Bowser MT (2011) Methods for measuring aptamer-protein equilibria: a review. Anal Chim Acta 686:9–18
Nguyen TH, Steinbock LJ, Butt HJ, Helm M, Berger R (2011) Measuring single small molecule binding via rupture forces of a split aptamer. J Am Chem Soc 133:2025–2027
Taylor JN, Darugar Q, Kourentzi K, Willson RC, Landes CF (2008) Dynamics of an anti-VEGF DNA aptamer: a single-molecule study. Biochem Biophys Res Commun 373:213–218
Yangyuoru PM, Dhakal S, Yu Z, Koirala D, Mwongela SM, Mao H (2012) Single-molecule measurements of the binding between small molecules and DNA aptamers. Anal Chem 84:5298–5303
Berezovski M, Krylov SN (2002) Nonequilibrium capillary electrophoresis of equilibrium mixtures–a single experiment reveals equilibrium and kinetic parameters of protein-DNA interactions. J Am Chem Soc 124:13674–13675
Drabovich AP, Berezovski M, Okhonin V, Krylov SN (2006) Selection of smart aptamers by methods of kinetic capillary electrophoresis. Anal Chem 78:3171–3178
Tasset DM, Kubik MF, Steiner W (1997) Oligonucleotide inhibitors of human thrombin that bind distinct epitopes. J Mol Biol 272:688–698
Kanaseki T, Kawasaki K, Murata M, Ikeuchi Y, Ohnishi S (1997) Structural features of membrane fusion between influenza virus and liposome as revealed by quick-freezing electron microscopy. J Cell Biol 137:1041–1056
Acknowledgments
This work was supported by the project funded by Agricultural Finance, Ministry of Agriculture of the People’s Republic of China.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 118 kb)
Rights and permissions
About this article
Cite this article
Zhang, YW., Yan, HY., Fu, P. et al. Modified capillary electrophoresis based measurement of the binding between DNA aptamers and an unknown concentration target. Anal Bioanal Chem 405, 5549–5555 (2013). https://doi.org/10.1007/s00216-013-6968-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6968-0