Abstract
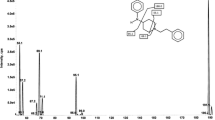
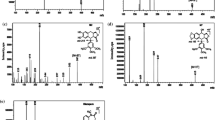
The benzodiazepine midazolam is a probe drug used to phenotype cytochrome P450 3A activity. In this situation, effective sedative concentrations are neither needed nor desired, and in fact the use of very low doses is advantageous. We therefore developed and validated an assay for the femtomolar quantification of midazolam and 1′-hydroxymidazolam in human plasma. Plasma (0.25 mL) and 96-well-based solid-phase extraction were used for sample preparation. Extraction recoveries ranged between 75 and 92% for both analytes. Extracts were chromatographed within 2 min on a Waters BEH C18 1.7 μm UPLC® column with a fast gradient consisting of formic acid, ammonia, and acetonitrile. Midazolam and 1′-hydroxymidazolam were quantified using deuterium- and 13C-labeled internal standards and positive electrospray tandem mass spectrometry in the multiple reaction monitoring mode, which yielded lower limits of quantification of 50 fg/mL (154 fmol/L) and 250 fg/mL (733 fmol/L) and a corresponding precision of <20%. The calibrated concentration ranges were linear for midazolam (0.05–250 pg/mL) and 1′-hydroxymidazolam (0.25–125 pg/mL), with correlation coefficients of >0.99. Within-batch and batch-to-batch precision in the calibrated ranges for both analytes were <14% and <12%. No ion suppression was detectable, and plasma matrix effects were minimized to <15% (<25%) for midazolam (1′-hydroxymidazolam). The assay was successfully applied to assess the kinetics of midazolam in two human volunteers after the administration of single oral microgram doses (1–100 μg). This ultrasensitive assay allowed us to quantify the kinetics of midazolam and 1′-hydroxymidazolam for at least 10 h, even after the administration of only 1 μg of midazolam.

UPLC/MS/MS quantification of femtomolar midazolam plasma concentrations





Similar content being viewed by others
References
Guengerich FP (2003) Cytochromes P450, drugs, and diseases. Mol Interv 3:194–204
Michalets EL (1998) Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 18:84–112
Breimer DD, Schellens JH (1990) A “cocktail” strategy to assess in vivo oxidative drug metabolism in humans. Trends Pharmacol Sci 11:223–225
Christensen M, Andersson K, Dalén P, Mirghani RA, Muirhead GJ, Nordmark A, Tybring G, Wahlberg A, Yaşar U, Bertilsson L (2003) The Karolinska cocktail for phenotyping of five human cytochrome P450 enzymes. Clin Pharmacol Ther 73:517–528
Zhou H, Tong Z, McLeod JF (2004) “Cocktail” approaches and strategies in drug development: valuable tool or flawed science? J Clin Pharmacol 44:120–134
Chainuvati S, Nafziger AN, Leeder JS, Gaedigk A, Kearns GL, Sellers E, Zhang Y, Kashuba ADM, Rowland E, Bertino JS (2003) Combined phenotypic assessment of cytochrome P450 1A2, 2C9, 2C19, 2D6, and 3A, N-acetyltransferase-2, and xanthine oxidase activities with the “Cooperstown 5 + 1 cocktail.” Clin Pharmacol Ther 74:437–447
Katzenmaier S, Markert C, Riedel K-D, Burhenne J, Haefeli WE, Mikus G (2011) Time course of CYP3A inhibition by potent reversible (voriconazole) and irreversible CYP3A inhibitors (ritonavir) using a limited sampling strategy. Clin Pharmacol Ther (in press)
Spina SP, Ensom MH (2007) Clinical pharmacokinetic monitoring of midazolam in critically ill patients. Pharmacotherapy 27:389–398
Kaartama R, Jarho P, Savolainen J, Kokki H, Lehtonen M (2011) Determination of midazolam and 1-hydroxymidazolam from plasma by gas chromatography coupled to methane negative chemical ionization mass spectrometry after sublingual administration of midazolam. J Chromatogr B 879:1668–1676
Tiscione NB, Shan X, Alford I, Yeatman DT (2008) Quantitation of benzodiazepines in whole blood by electron impact–gas chromatography–mass spectrometry. J Anal Toxicol 32:644–652
Papoutsis II, Athanaselis SA, Nikolaou PD, Pistos CM, Spiliopoulou CA, Maravelias CP (2010) Development and validation of an EI-GC-MS method for the determination of benzodiazepine drugs and their metabolites in blood: applications in clinical and forensic toxicology. J Pharm Biomed Anal 52:609–614
Simonsen KW, Hermansson S, Steentoft A, Linnet K (2010) A validated method for simultaneous screening and quantification of twenty-three benzodiazepines and metabolites plus zopiclone and zaleplone in whole blood by liquid–liquid extraction and ultra-performance liquid chromatography–tandem mass spectrometry. J Anal Toxicol 34:332–341
Gunn J, Kriger S, Terrell AR (2010) Simultaneous determination and quantification of 12 benzodiazepines in serum or whole blood using UPLC/MS/MS. Methods Mol Biol 603:107–119
Marin SJ, Coles R, Merrell M, McMillin GA (2008) Quantitation of benzodiazepines in urine, serum, plasma, and meconium by LC-MS-MS. J Anal Toxicol 32:491–498
Turpault S, Brian W, van Horn R, Santoni A, Poitiers F, Donazzolo Y, Boulenc X (2009) Pharmacokinetic assessment of a five-probe cocktail for CYPs 1A2, 2C9, 2C19, 2D6 and 3A. Br J Clin Pharmacol 68:928–935
Zhang W, Han F, Guo P, Zhao H, Lin ZJ, Huang MQ, Bertelsen K, Weng N (2010) Simultaneous determination of tolbutamide, omeprazole, midazolam and dextromethorphan in human plasma by LC-MS/MS—a high throughput approach to evaluate drug–drug interactions. J Chromatogr B 878:1169–1177
Wohlfarth A, Naue J, Lutz-Bonengel S, Dresen S, Auwärter V (2011) Cocktail approach for in vivo phenotyping of 5 major CYP450 isoenzymes: development of an effective sampling, extraction, and analytical procedure and pilot study with comparative genotyping. J Clin Pharmacol (in press)
Ghassabian S, Chetty M, Tattam BN, Glen J, Rahme J, Stankovic Z, Ramzan I, Murray M, McLachlan AJ (2009) A high-throughput assay using liquid chromatography–tandem mass spectrometry for simultaneous in vivo phenotyping of 5 major cytochrome P450 enzymes in patients. Ther Drug Monit 31:239–246
Ardjomand-Woelkart K, Kollroser M, Li L, Derendorf H, Butterweck V, Bauer R (2011) Development and validation of a LC-MS/MS method based on a new 96-well hybrid-SPE-precipitation technique for quantification of CYP450 substrates/metabolites in rat plasma. Anal Bioanal Chem 400:2371–2381
Svanström C, Hansson GP, Svensson LD, Sennbro CJ (2011) Development and validation of a method using supported liquid extraction for the simultaneous determination of midazolam and 1′-hydroxy-midazolam in human plasma by liquid chromatography with tandem mass spectrometry. J Pharm Biomed Anal (in press)
Dostalek M, Macwan JS, Chitnis SD, Ionita IA, Akhlaghi F (2010) Development and validation of a rapid and sensitive assay for simultaneous quantification of midazolam, 1′-hydroxymidazolam, and 4-hydroxymidazolam by liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B 878:1629–1633
De Loor H, De Jonge H, Verbeke K, Vanrenterghem Y, Kuypers DR (2010) A highly sensitive liquid chromatography tandem mass spectrometry method for simultaneous quantification of midazolam, 1′-hydroxymidazolam and 4-hydroxymidazolam in human plasma. Biomed Chromatogr 25:1091–1098
US Department of Health and Human Services FDA (2001) Guidance for industry, bioanalytical method validation. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf. Accessed 13 Dec 2011
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75:3019–3030
Link B, Haschke M, Wenk M, Krähenbühl S (2007) Determination of midazolam and its hydroxyl metabolites in human plasma and oral fluid by liquid chromatography/electrospray ionization ion trap tandem mass spectrometry. Rapid Commun Mass Spectrom 21:1531–1540
Li W, Luo S, Smith HT, Tse FLS (2007) Simultaneous determination of midazolam and 1′-hydroxymidazolam in human plasma by liquid chromatography with tandem mass spectrometry. Biomed Chromatogr 21:841–851
Annesley TM (2003) Ion suppression in mass spectrometry. Clin Chem 49:1041–1044
Bonfiglio R, King RC, Olah TV, Merkle K (1999) The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun Mass Spectrom 13:1175–1185
Greenblatt DJ, Peters DE, Oleson LE, Harmatz JS, MacNab MW, Berkowitz N, Zinny MA, Court MH (2009) Inhibition of oral midazolam clearance by boosting doses of ritonavir, and by 4,4-dimethyl-benziso-(2H)-selenazine (ALT-2074), an experimental catalytic mimic of glutathione oxidase. Br J Clin Pharmacol 68:920–927
Lappin G, Kuhnz W, Jochemsen R, Kneer J, Chaudhary A, Oosterhuis B, Drijfhout WJ, Rowland M, Garner RC (2006) Use of microdosing to predict pharmacokinetics at the therapeutic dose: experience with 5 drugs. Clin Pharmacol Ther 80:203–215
Acknowledgement
The authors would like to thank Dr. Markus Winkler, Dr. Gislinde Gundel, and Dr. Michael Kudlich from Waters Corporation (Eschborn/Germany) for technical input, and Andrea Deschlmayr and Magdalena Longo for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burhenne, J., Halama, B., Maurer, M. et al. Quantification of femtomolar concentrations of the CYP3A substrate midazolam and its main metabolite 1′-hydroxymidazolam in human plasma using ultra performance liquid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem 402, 2439–2450 (2012). https://doi.org/10.1007/s00216-011-5675-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5675-y