Abstract
Cigarette smoke is a complex mixture of smoke constituents, often characterised by size-resolved particle distributions. Since descriptions of ultrafine particles <50 nm are absent, our aim was to explore the existence of these nanoparticles in fresh and undiluted cigarette smoke. We measured undiluted smoke particles real-time by a scanning mobility particle sizer with Faraday cup electrometer, integrated in our custom-made smoking machine. Cigarettes were smoked by 2 s puffs, 30 s puff intervals and 50 ml puff volume. We tested six different cigarettes (1–10 mg tar per cigarette) at ten particle size-ranges between 6 and 50 nm, and repeated measurements five times. The formation of nanoparticles in fresh cigarette smoke was observed over the entire range between 6 and 50 nm, and reproduced in all cigarettes. The highest mean yield was 8.8 × 109 (SD = 1.1 × 109) particles per cigarette at the largest particle size range by high-tar cigarettes. Nanoparticle counts appear to increase with particle size, claimed tar values and blocking of filter ventilation holes, and inversely with butt length. Fresh undiluted cigarette smoke contains large amounts of potentially toxic nanoparticles <50 nm. We recommend to further study nanoparticles in the characterisation of cigarette smoke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cigarette smoke is a complex mixture of potentially pathogenic smoke constituents, including particulate matter, volatile substances and gasses. Several techniques that characterise cigarette smoke have been reviewed during the last century, including the current method to categorise cigarettes by tar, nicotine and carbon monoxide content [1, 2, 3]. Techniques that analyse smoke constituents more specifically and usually in relation to tar yields include single constituent analyses [1, 4, 5, 6, 7] and size-resolved particle distributions by different spectrometry methods [8, 9, 10, 11, 12].
Limitations of measurement techniques include low time resolution, aerosol (ageing) dynamics and high particle concentrations, which cause the need for (possibly time-delaying) dilution [1, 8]. Particles consequently agglomerate substantially before measurement, apart from evaporation, condensation, diffusion and (gravitational) sedimentation [1]. Mainly due to these limiting measurement factors, until recently, only two reports have been published of smoke particles below 100 nm in diameter [9, 10]. The first used orifices of 0.5 mm whereas the other needed a dilution of 80,000:1, which are both important limitations. Recently, nanoparticles have been studied more extensively by real-time size-resolved particle distributions for instance by electrical low-pressure impactor and differential electrical mobility particle spectrometry [8, 12, 13, 14]. These techniques are able to measure particles down to 5 nm. Both techniques however implicated dilutions of 50:1 and 750:1 and time delays range from 0.2 s to 1.5 s. Furthermore, the related studies only report the count median diameter and do not explicitly study nanoparticles. Some figures however do reveal the existence of nanoparticles upward of 70 nm [8, 12]. Still, little is known about particles smaller than 70 nm. Nanoparticles either are very difficult to measure with the available techniques or are only scarce. As particles are abundant above 100 nm and as the largest amount of cigarette smoke has been reported to result from gasses with diameters of around 0.5 nm [1], one might consequently expect large quantities of particles within the size-range between 1 and 100 nm. The existence of nanoparticles in cigarette smoke would be quite relevant as they likely have a higher deposition and transposition and thus a higher toxic potency [15, 16].
We hypothesise the existence of large quantities of nanoparticles above 5 nm. The Scanning Mobility Particle Sizer with Faraday Cup Electrometer (SMPS + E), by Grimm Aerosol Technik, measures concentrations up to 108 particles per cm3 and is able to detect nanoparticles from 6 to 1,100 nm real-time. SMPS + E measurements are based on size-specific electrical loading of particles and subsequently size-specific electrical and mechanical mobility of particles. The SMPS + E appears to have promising qualities in this, so far, poorly explored particle size range within cigarette smoke.
Our aim in this study is to explore this existence of nanoparticles in fresh unaged and undiluted cigarette smoke, and to determine their contribution to human cigarette smoke exposure, i.e., their contribution to human health-hazard. Since filter ventilation is a very important factor in altering (particle size-resolved) smoke yields, [8, 12, 17] by dilution and by altering combustion, we also explored the influence of filter ventilation on the appearance of nanoparticles.
Methods
Study design
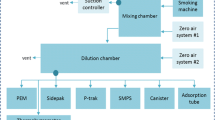
The main levels that influence smoke measurements include production, collection and measurement itself. We standardised smoke production by a custom-made smoking machine, integrated in our SMPS + E (Fig. 1), which consequently standardised the smoke collection and allowed standardisation of the measurements. By a pilot study (not reported), we adjusted the smoking regime and measurement settings so we ended up with a standardised method that produced typical and valid smoking interval-dependent particle counts [12]. We selected a 2-s puff, 30-s interval, 50-ml puff volume, and ten continuous 8-Hz single channel measurements, with geometric mean particle diameters between 5.65 and 50.38 nm at 5-nm intervals. Each channel covers a size range of approximately 1 nm. We measured six cigarettes with different filter ventilation capacities and different determined tar mass yields, and each cigarette measurement was repeated five times: three cigarettes manufactured by Phillip Morris and three by British American Tobacco as displayed in Table 1.
Smoke production
Smoking was based on the constant air-flow of the spectrometer, which was connected to the 4-mm valve outlet by inert carbosilicon tubing. The setting included a two-way valve with two 4-mm inlet orifices that allowed smoking through the orifice with the cigarette attached and allowed a smoking interval through the other orifice with clean room air attached. A timer that managed the valve direction could subsequently adjust puff duration and frequency. We ignited cigarettes electrically and smoked cigarettes up to 32 mm from the filter end. All cigarettes were smoked with their filters inserted 2 cm in the filter holder. The three cigarettes manufactured by Phillip Morris were also smoked with their filters inserted 1 cm and their main filter ventilation holes uncovered. Cigarettes were stored beforehand at 22 °C and at 60% relative humidity for at least 2 days.
Study outcome
We defined the size-resolved particle distribution per smoked cigarette as our outcome. Particle distributions are presented as lognormal size distributions: dN/dln(D) per ml produced smoke—dN equals particle count, dln(D) equals lognormal channel size range [18]. For each channel, the total particle count of one cigarette measurement was derived from area under the curve calculations from all successive 2 s puff periods. We multiplied the mean particle concentration per puff by 50, to attain the particle number per 50-ml puff volume. We calculated the mean total particle count of five different measurements.
Analysis
We analysed mean channel particle counts, including standard deviation (SD) and coefficients of variation. Test of normality for distributions was performed by Shapiro–Wilk (p < 0.05) [19]. We compared particle counts of different cigarette types and filter ventilation blocking conditions, and performed analysis of variance (ANOVA) for each separate channel. We used SPSS 16.0 for all analyses.
Results
Filters inserted 2 cm
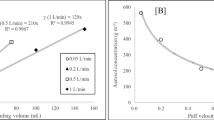
By evaluating the interval-dependent particle counts, we observed valid smoke peaks lasting 1/8 to 2 s at all channels for all six cigarettes. However, at 46 and 50 nm, some puffs attained the maximum concentration measurable, particularly in high-tar cigarettes. Particle counts per puff were higher at smaller butt lengths, due to an increase of both the peak height and duration (Fig. 2). Coefficients of variation of total particle counts per cigarette ranged from 5% to 92% (mean 22%) for different channels. Tests of normality revealed four non-parametric distributions out of 60 tests.
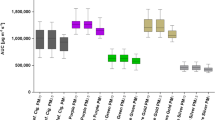
For cigarettes manufactured by Phillip Morris, the lowest particle yield was at 6 nm by Philip Morris One, 2.6 × 106 (SD 1.2 × 106) particles per cigarette, whereas the highest yield was at 50 nm by Marlboro Red, 8.8 × 109 (SD = 1.1 × 109) particles per cigarette. We observed that particle counts increased parallel to particle diameter size similarly across different cigarette types (Fig. 3). Although particle counts differed significantly between different cigarette types (Table 2), they did not differ much below 40 nm, whereas above 40 nm the highest tar cigarette revealed excessive particle yields due to a very rapid increase.
We observed the lowest particle yield in Kent cigarettes at 6 nm by Kent Infina, 3.0 × 106 (SD 9.4 × 105) particles per cigarette, and the highest yield at 50 nm by Kent Futura, 5.8 × 109 (SD 1.3 × 109) particles per cigarette (Fig. 4). Again, particle yields increased similarly along with particle diameter size across the different cigarettes, with highest yields for the highest tar cigarette (Kent Futura), significant at most channels. However, differences were relatively small compared to claimed tar mass yields.
Filters inserted 1 cm
Three cigarettes were also tested with unblocked filter ventilation holes as well. For these cigarettes, we observed valid smoke peaks only at particle sizes upward of 30 nm, based on the shapes and height of the smoke peaks, peak intervals, and the standard deviations of the particle counts per cigarette. Figure 5 shows an example of particle numbers measured at 41 nm. Below 30 nm, these peaks appeared scarce and with random intervals, and relatively large standard deviations. Between 30 and 50 nm coefficients of variation ranged from 9% to 101% (mean 49%). Tests of normality revealed two non-parametric distributions out of 15 tests. Particle counts per puff again were higher at smaller butt lengths, increased along with particle diameter size, and appeared to be reflected by claimed tar mass yields (Fig. 6). Compared to smoking with blocked filter ventilation holes, the yields of all cigarettes tended to decrease about tenfold at each channel, but mainly affected Philip Morris One cigarettes with high filer ventilation capacity.
Discussion
Although previous studies on cigarette smoke did not explicitly report on nanoparticles between 6 and 50 nm, our study reveals their existence in fresh and undiluted smoke, from cigarettes smoked by 2-s puffs, 30-s intervals and 50-ml puff volumes. We managed to reproduce the results in different cigarette types and by different filter ventilation blocking. For a single channel measurement, the highest mean yield was 8.8 × 109 (SD = 1.1 × 109) particles per cigarette.
Nanoparticle counts appear to increase with particle size, claimed tar values and blocking of filter ventilation holes, and inversely with butt length. Although high-tar cigarettes seem to have an overall excess of particles compared to lower tar cigarettes, the channel-based ratios between the particle yields vary.
Validation
Variation of size-dependent particle counts within a certain cigarette appear comparable to the variations of tar measurements within a certain cigarette. Apparently, most of our observed variation is due to natural variations within the cigarettes themselves. In addition, our results revealed smoking interval-dependent particle counts, and the increase of particle counts for successive puffs corresponds to the results of prior studies [11, 8]. Alas, at two channels—46 and 50 nm—we sometimes attained the concentration threshold, particularly in high-tar cigarettes. Furthermore, in our pilot study, we observed a lower limit of necessary smoking intensity that suggests nanoparticles require combustion circumstances that not necessarily always occur during real-life smoking. Altogether, we believe our method is valid in measuring fresh and undiluted cigarette smoke, though the two largest channels might underestimate the particle counts in high-tar cigarettes.
Interpretation
Although the interpretation of our results appears confined to our smoking regime and selection of cigarettes, the possibility to generalise our results is tempting. Since our smoking regime is moderate compared to other common used regimes [1] and our selection of cigarettes cover a wide range of cigarette types, we assume some general remarks on our results may be allowed.
Although we hypothesised high particle counts, compared to previous studies that report size-resolved particle distributions in fresh cigarette smoke, the contribution of our encountered nanoparticles would be only a few percent [8]. Even more, the particle counts decreased for smaller particles. Possibly, the small nanoparticles have a high potency to quickly agglomerate into larger smoke constituents or to disperse into smaller molecules due to their volatile properties. In addition, the increase of nanoparticle counts due to smaller butt lengths, also suggests crucial time-dependent agglomeration and evaporation in successive puffs, apart from improved combustions and decreased tobacco-dependent filter potency, which has already been observed for larger particles [8, 12]. However, by a possibly increased deposition in and transposition through the pulmonary tissue, the probably excessive toxic properties of these nanoparticles would still indicate a substantial contribution in health-hazard [15, 16].
Overall, the particle yields seem to depend on claimed tar values, with higher yields for higher-tar cigarettes. Although the particle counts only roughly reflect the size resolved particle distributions, it suggests an overall enhanced combustion and increased production of all particles for higher-tar cigarettes but maybe also a different combustion with different size-resolved particle distributions.
Interpretation of (blocking of) filter ventilation holes was only possible for channels ≥ 30 nm. However, a more intense regime by ventilation blocking seems to excessively increase nanoparticle counts in both high-ventilated and low-ventilated filters. Although an excess of particles by vent-blocking—as measured by tar yields and carbon monoxide—has already been observed particularly in high-ventilation cigarettes, [20] the production of nanoparticles is influenced substantially by low-ventilation as well. Otherwise, we might have encountered a tipping point for combustion requirements. In addition, the differences between Philip Morris One and Marlboro Red cigarettes were smaller when ventilation holes were blocked, which equalises dilution and combustion circumstances across different cigarette types. The remaining differences in particle yield however, indicate that other factors like porous cigarette papers, expanded tobacco and reconstituted tobacco sheet, have a substantial influence on nanoparticles as well. These effects seem to be more pronounced on the formation of nanoparticles than expected from their effects on tar yields [21].
In conclusion, when cigarettes are smoked by moderate smoking regimes, fresh and undiluted cigarette smoke contains an abundance of nanoparticles, which has not been described previously. These particles are present in such amounts and likely have relatively high toxic properties. Therefore, a substantial toxic effect on human health can be expected. The production of nanoparticles requires a minimum smoking intensity, and is related to particle size, filter ventilation holes, butt length, and claimed tar values. We suggest to further study the necessity of measuring these particles in the smoke characterisation of different cigarettes.
References
Borgerding M, Klus H (2005) Exp Toxicol Pathol 57(Suppl 1):43–73
Philip Morris International Management SA. www.philipmorrisinternational.com, accessed 2010.
Baker RR (2002) Beiträge zur Tabakforschung International 20:23–41
Chepiga TA, Morton MJ, Murphy PA, Avalos JT, Bombick BR, Doolittle DJ, Borgerding MF, Swauger JE (2000) Food Chem Toxicol 38:949–962
Gendreau PL, Vitaro F (2005) Can J Public Health 96:167–172
Gregg E, Hill C, Hollywood M, Kearney M, McAdam K, McLaughlin D, Purkis S, and Williams M (2004) Beiträge zur Tabakforschung International/Contributions to Tobacco Research 21:117–138.
D. Hoffmann. Analysis of toxic smoke constituents. In: Toxicity testing plan vol. 5. 1993.
Adam T, McAughey J, McGrath C, Mocker C, Zimmermann R (2009) Anal Bioanal Chem 394:1193–1203
Ingebrethsen BJ (1986) Aerosol Sci Technol 5:423–433
Anderson PJ, Wilson JD, Hiller FC (1989) Am Rev Respir Dis 140:202–205
Bernstein DM (2004) Inhal Toxicol 16:675–689
Kane DB, Asgharian B, Price OT, Rostami A, Oldham MJ (2010) Inhal Toxicol 22:199–209
Dickens C, McGrath C, Warren N, Biggs P, McAughey J (2009) J Phys Conf Ser 151:012019
McGrath C, Warren N, Biggs P, McAughey J (2009) J Phys Conf Ser 151:012018
Jaques PA, Kim CS (2000) Inhal Toxicol 12:715–731
Schmid O, Möller W, Semmler-Behnke M, Ferron GA, Karg E, Lipka J, Schulz H, Kreyling WG, Stoeger T (2009) Biomarkers 14:67–73
Becquemin MH, Bertholon JF, Attoui M, Roy F, Roy M, Dautzenberg B (2007) Rev Mal Respir 24:845–852
Reischl GP (1991) J Aerosol Sci 22:297–312
Shapiro SS, Wilk MB (1965) Biometrika 52:591–611
Kozlowski LT, O’Connor RJ (2002) Tob Control 11(Suppl 1):I40–I50
O’Connor RJ, Hammond D, McNeill A, King B, Kozlowski LT, Giovino GA, Cummings KM (2008) Tob Control 17(Suppl 1):i1–i5
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Dijk, W.D., Gopal, S. & Scheepers, P.T.J. Nanoparticles in cigarette smoke; real-time undiluted measurements by a scanning mobility particle sizer. Anal Bioanal Chem 399, 3573–3578 (2011). https://doi.org/10.1007/s00216-011-4701-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-4701-4