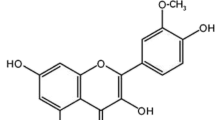
Abstract
A rapid, ultra high-performance liquid chromatographic (UHPLC) method has been developed and validated for simultaneous identification and analysis of the isoflavones genistein, daidzein, glycitin, puerarin, and biochanin A, and the flavonoids (±)-catechin, (−)-epicatechin, rutin, hesperidin, neohesperidin, quercitrin, and hesperetin in human urine. Urine samples were incubated with β-glucuronidase/sulfatase. UHPLC was performed with a Hypersil Gold (50 × 2.1 mm, 1.9 μm) analytical column. Elution was with a gradient prepared from aqueous trifluoroacetic acid (0.05%) and acetonitrile. UV detection was performed at 254 and 280 nm. The calibration curves were indicative of good linearity (r 2 ≥ 0.9992) in the range of interest for each analyte. LODs ranged between 15.4 and 107.0 ng mL−1 and 3.9 and 20.4 ng mL−1 for flavonoids and isoflavones, respectively. Intra-day and inter-day precision (C.V., %) was less than 3.9% and 3.8%, respectively, and accuracy was between 0.03% and 5.0%. Recovery was 70.35–96.58%. The method is very rapid, simple, and reliable, and suitable for pharmacokinetic analysis. It can be routinely used for simultaneous determination of these five isoflavones and seven flavonoids in human urine. The method can also be applied to studies after administration of pharmaceutical preparations containing isoflavones and flavonoids to humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, much attention has been devoted to natural substances, for example isoflavones and flavonoids, with antioxidant activity. These natural antioxidants are mainly found in vegetables, fruit, and wheat [1]. Interest in these compounds is because of the increasing incidences of serious pathologies, for example cancer, cardiovascular diseases, or inflammation, caused, in part, by the harmful effects of free radicals. There is strong epidemiological evidence that diet has a protective effect against a number of these complex diseases; thus, there is an increased interest in natural antioxidant compounds [2–4].
Therefore, a daily diet that includes foods and food products containing antioxidants is important to health and nutrition. Isoflavones and flavonoids are biologically active phytochemicals that humans are exposed to mainly through food intake. Flavonoids are found in nearly every plant. However, isoflavones are usually treated separately from other subclasses of antioxidants because these phytochemicals are found in significant concentrations chiefly in soybeans and soy-related foods. Interest in supplementing the diet with isoflavones and flavonoids is constantly increasing. These compounds are available as various pharmaceutical preparations that are currently being recommended by doctors.
In order to probe the potential benefits, or adverse effects, of isoflavone and flavonoid consumption more efficiently, it is necessary to develop analytical methods capable of sensitive and accurate quantification of a number of analytes in low volumes of human fluids. These methods should also be capable of high-throughput sample analysis. Different methods have been reported in the literature for analysis of individual flavonoids or isoflavones; however, there is no literature that describes the simultaneous determination of isoflavones and flavonoids by ultra high-performance liquid chromatography (UHPLC).
HPLC, with various types of detectors, for example ultra-violet (UV) [5–9], diode array [10], coulometric array [11], and mass spectrometry [12–14]), is the most common method used for determination of flavonoids in biological fluids. Flavonoids have been quantified in the presence of other antioxidants using electrochemical detection by Bolarinwa and Linseisen [15]. Papers have been published on flavonoid separation using different mobile phases; most separations have been accomplished on C8 or C18 reversed-phase columns.
Measurements of isoflavones in biological samples have been conducted using gas chromatography–mass spectrometry (GC–MS) [16, 17] or HPLC, usually with UV [18–20], electrochemical [21, 22], or mass spectrometric [23–26] detection. Methods such as liquid–liquid extraction (LLE) or solid-phase extraction (SPE) have been used for isoflavone extraction and purification, because biological samples require clean up [27, 28].
Recently, commercially available UHPLC has proved to be one of the most promising developments in fast chromatographic separations. There has been substantial focus on UHPLC separations with the objective of reducing analysis times while maintaining good efficiency. In this work, a new gradient reversed-phase UHPLC method was developed for simultaneous analysis of the isoflavones genistein (GT), daidzein (DA), glycitin (GLY), puerarin (PUR), and biochanin A (BIO) and the flavonoids (±)-catechin ((±)-CA), (−)-epicatechin ((−)-EC), rutin (RUT), hesperidin (HSD), neohesperidin (NHSD), quercitrin (QUR), and hesperetin (HST). The development of new and highly effective techniques for isolation, separation, identification, and determination of isoflavones and flavonoids is very important because these substances are known to have very positive physiological effects on human health. As far as we are aware, there is no literature related to the simultaneous analysis of isoflavones and flavonoids in biological samples. This newly developed method is applicable for the analysis of human urine.
Experimental
Reagents and chemicals
The flavonoids (±)-CA, (−)-EC, RUT, HSD, NHSD, QUR, and HST, the isoflavones GT, DA, GLY, PUR, and BIO, and β-glucuronidase/sulfatase (crude solution from Helix pomatia, type HP-2, G7017) were purchased from Sigma Chemicals (St Louis, MO, USA) and Aldrich Chemicals (Milwaukee, WI, USA). HPLC-grade acetonitrile, water, trifluoroacetic acid, formic acid, sodium acetate buffer (pH 4.66), and phosphate buffer (pH 2.4) were obtained from Merck (Darmstadt, Germany). Analytical-grade methanol, acetonitrile, and acetone were purchased from POCh (Gliwice, Poland).
Standard stock solutions (1 mg mL−1) of the flavonoids and isoflavones were prepared in methanol as solvent. Combined standard solutions containing the flavonoids and isoflavones were prepared just before use by mixing individual stock solutions and diluting these mixtures with methanol. All solutions were stored at 4 °C. In this study an internal standard was not used.
Human urine was obtained from healthy volunteers who were on a diet rich in flavonoids and orally-administrated tablets of flavonoids and isoflavones. Human urine samples taken from patients treated with β-blockers were obtained from the Medical University in Katowice (Poland). The subjects were ten healthy volunteers aged 21–48 years. Urine samples were collected just before administration of pharmaceutical preparations containing isoflavones and flavonoids and 0–2, 2–4, 4–6, 6–8, 8–10, 10–12, and 12–24 h after administration. Human urine was stored at −18 °C.
Chromatographic conditions
The UHPLC system (Merck Hitachi, Germany) was equipped with a model L-2160U pump, a model L-2400U absorbance detector, a model L-2200U autosampler, a model L-2350U thermostatted column compartment, and a degasser module.
The flavonoids and isoflavones were separated on a Hypersil Gold (50 × 2.1 mm, 1.9 μm) reversed-phase column (Thermo Scientific) by gradient elution using 0.05% trifluoroacetic acid (TFA) in water (solvent A) and acetonitrile (solvent B). The gradient profile was: 0–0.5 min, from 5% to 20% solvent B (flow rate from 1.0 to 0.5 mL min−1); 2.0 min, 40% solvent B (flow rate 0.4 mL min−1); 3.5 min 90% solvent B (flow rate 0.9 mL min−1). The detector wavelengths were set at 254 and 280 nm. The injection volume was 2 μL. The temperature of the column oven was set to 25 °C.
Isoflavone and flavonoid identification was performed according to retention time by comparison with reference standards. Furthermore, the presence of the individual flavonoids and isoflavones in the urine matrix was confirmed by adding a mixed standard solution to the urine samples. Data acquisition and integration were performed using EZ Chrom Elite System Manager.
Sample processing
Urine samples were collected from healthy volunteers after oral administration of tablets containing flavonoids and isoflavones for a period of 1 h. For sample preparation, 2.5 mL urine was mixed with 0.5 mL acetonitrile and 0.5 mL methanol. After shaking for 1 min, the sample was transferred to a centrifuge tube and centrifuged using a refrigerated Universal Centrifuge Z 323 K (Hermle Labortechnik, Germany) at 4724 × g for 15 min at room temperature (ca. 22 °C). The clear supernatant was incubated with 100 μL 1 mol L−1 sodium acetate buffer (pH 4.66) and 40 μL β-glucuronidase/sulfatase (crude preparation from H. pomatia) for 18 h at 37 °C. The hydrolyzed urine sample was diluted with 1 mL phosphate buffer (0.1 mol L−1, pH 2.4) [7].
Extraction was performed with the Bakerbond SPE-12G system (J.T. Baker, Deventer, Netherlands). The hydrolyzed sample was applied to Oasis HLB extraction cartridges (6 mL, 500 mg, Waters), preconditioned successively with 6 mL methanol and 6 mL 0.1% formic acid, and allowed to run through. Isoflavones and flavonoids were eluted with 5 mL methanol–acetone–formic acid 4.5:4.5:1 (v/v) and the eluate was evaporated to dryness. Finally, the residue was redissolved in 1 mL 0.05% trifluoroacetic acid (TFA) in water and filtered through a 0.45-μm membrane filter. Samples (2 μL) were injected on to the chromatographic column using the system autosampler. The analysis was repeated six times.
Pharmacokinetic analysis
Pharmacokinetic analysis was carried out using PK Solutions 2.0 pharmacokinetic analysis computer software. The area under the curve (AUC), maximum plasma concentration (C max), and time needed to reach the maximum plasma concentration (T max) were determined by the software. The elimination rate constant (K el) was obtained from the terminal slope by use of regression analysis. The half-life (t 1/2) of the drug was calculated by use of the relationship t 1/2 = 0.693/K el.
Method validation
The newly developed UHPLC method was validated for linearity, limit of detection (LOD), limit of quantification (LOQ), precision, accuracy, selectivity, and recovery.
Calibration standards were prepared by adding appropriate amounts of the stock solutions to flavonoid and isoflavone-free human urine and then performing serial dilutions with additional blank urine sample to obtain different concentrations. Six replicate aliquots of these solutions were injected on to the UHPLC system and the analyses were carried out as described in the section “Chromatographic conditions”. Calibration curves were constructed by plotting peak area against concentration for each analyte and the regression equation was calculated for each curve. The data obtained were submitted to regression analysis and correlation coefficients were calculated for each flavonoid and isoflavone using Excel.
The LOD and LOQ were calculated using Eqs. 1 and 2, respectively [29]. The calculated theoretical detection and quantification limits were confirmed by analysis of these concentrations using the developed method.
where S.D. is the standard deviation of the intercept.
The precision and accuracy of the UHPLC method were evaluated by analysing blank samples of urine spiked with high, medium, and low concentrations of the flavonoids and isoflavones. The intra-day variance was determined by assaying six replicates on the same day and inter-day variance was assessed over five consecutive days. Precision was expressed as relative standard deviation (C.V., %). Accuracy was determined by comparing the calculated concentrations from the calibration curves with the known concentrations.
Control human urine obtained from six volunteers, was assessed using the procedure described above and compared with results from respective urine samples to evaluate the selectivity of the method. The resulting chromatograms were examined to determine the presence of any peak that could interfere with the analysis of flavonoids and isoflavones.
Absolute recovery was calculated by injecting urine spiked with the known amounts of each examined compound. The recoveries were tested at low, medium, and high concentrations. The spiked urine samples were extracted using the described SPE method and analysed with the proposed UHPLC method. Furthermore, the concentrations of flavonoids were calculated using the calibration curves. The recovery was calculated by comparing the determined amounts for the extracted urine samples with the known amounts added.
Results and discussion
Optimization of the UHPLC method
In this study a UHPLC method with UV detection is reported for quantification of flavonoids and isoflavones in human urine. Analyses were performed at 254 nm (RUT, QUR, PUR, GLY, DA, GT, BIO) or 280 nm ((±)-CA, (−)-EC, HSD, NHSD, HST), which are the wavelengths corresponding to the absorption maximum for most of the flavonoids and isoflavones.
Various columns were tested to separate the flavonoids and isoflavones. It was determined that these compounds can be separated by using a Hypersil Gold (50 × 2.1 mm, 1.9 μm) column. However, peak tailing and peak broadening of the flavonoids and isoflavones occurred with this column. The separation is complicated by the fact that biological samples consist of many compounds with different properties. Some sample components are highly polar whereas others are non-polar. Different organic solvent mixtures (e.g., methanol–water or acetonitrile–water mixtures) were tried but the separation remained unsatisfactory. With a methanol–water mobile phase, the elution time of the flavonoids and isoflavones was prolonged and their separation was improved.
In this research, we studied the effects of different organic acids in the mobile phase (solvent A: trifluoroacetic (TFA), acetic, or formic acid; solvent B: acetonitrile) on the separation of the flavonoids and isoflavones. Basic chromatographic performance data, for example resolution (R S), selectivity (α), peak asymmetry, and peak area, were selected to evaluate the UHPLC method. The resolution and peak asymmetries of the flavonoids and isoflavones using different organic acids in the mobile phase are shown in Fig. 1a. Using acetic acid and formic acid, we observed a large increase in peak asymmetry and large decrease in peak height for the flavonoids and isoflavones. The effect of the organic acid on the separation is directly dependent on the particular flavonoids and isoflavone. The best peak width (0.07 min), R S (3.62), and α (1.08) for the separated compounds was observed with TFA. On the basis of the average values of the chromatographic performance data, the most effective mobile phase combination was TFA (solvent A) and acetonitrile (solvent B).
The pH of the mobile phase markedly influences the chromatographic separation of many compounds; thus, we studied this effect on the separation of flavonoids and isoflavones by adjusting the pH of the TFA mobile phase. The tested pH values were 2.5, 3.5, 4.5, 5.5, and 6.0. The best peak height, peak asymmetry, and resolution values for flavonoid and isoflavone detection and separation were obtained by using the lowest pH mobile phase (Fig. 1b). Increasing the pH from 2.5 to 6.0 had several negative effects on the separation. Using TFA at pH 6.0 increased the peak width (9.5%), peak asymmetry (9.6%), and retention time. More importantly, it reduced the selectivity and resolution. In addition, we observed a large decrease in the flavonoid and isoflavone peak heights at pH 6.0. Therefore, the chromatographic conditions were further studied using 0.05% TFA, pH 2.5, as mobile phase A.
The effect of increasing the column temperature from 20 °C to 40 °C was evaluated using the Hypersil Gold column at the optimum chromatographic conditions. Temperatures higher than 35 °C had a negative effect on the separation and on peak symmetry (Fig. 1c). The higher temperature increased the average peak width (32.9%) and peak asymmetry (11.9%), and reduced the selectivity (1.7%) and resolution (10.9%). Increasing the temperature to 40 °C reduced the retention times that, in turn, further reduced the separation of the unresolved compounds. The signals for PUR and (−)-EC were still not well resolved. The peak heights of the flavonoids and isoflavones increased with increasing temperature, but the peak symmetry and efficiency decreased. We prefer better chromatographic separation (i.e., no co-eluting peaks) to improved peak height (sensitivity); thus we decided to operate at 25 °C.
The optimized chromatographic conditions for the separation of the flavonoids and isoflavones were: solvent A, 0.05% TFA in water (pH 2.5); solvent B, acetonitrile; column temperature, 25 °C. The gradient elution and flow rate conditions are described in the section “Chromatographic conditions”. With these conditions, the best peak shape and separation were achieved. It was shown that separation of the flavonoids and isoflavones could be achieved within 3.5 min. Chromatograms demonstrating the separation are presented in Fig. 2.
Method validation
The LOD and LOQ of all the compounds analysed are listed in Table 1. A six-point linearity curve was constructed for each analyte. Samples were quantified using the concentration–peak-area relationships and were calculated by use of regression analysis \( \left( {y = ax + b} \right) \). The minimum correlation coefficient of the analyte calibration curves was greater than 0.9992; the regression data are listed in Table 1.
Both intra-day and inter-day precision, as relative standard deviation (C.V., %), were lower than 3.90%. The accuracy of the method was investigated by measurement of recovery. The results obtained showed the method was accurate. The percentage bias for all calibration samples varied from 0.03% to 5.00%. The accuracy results are listed in Table 2.
Selectivity experiments were carried out using human urine samples that did not contain the target compounds. A chromatogram obtained by UHPLC from a blank sample of urine is shown in Fig. 3. There were no interfering peaks at retention times corresponding to the analysed flavonoids and isoflavones. There are some additional unidentified peaks in the chromatogram from the human urine samples, but these peaks do not interfere with the flavonoids and isoflavones of interest.
The percentage recoveries for all analysed compounds from spiked human urine were evaluated at low (0.40–0.80 μg mL−1), medium (0.75–2.00 μg mL−1) and high (1.20–8.00 μg mL−1) concentrations. A high absolute percentage recovery indicates that the method can be successfully used for the determination of the analysed flavonoids and isoflavones in human urine samples. The overall mean recoveries calculated for the flavonoids and isoflavones are shown in Table 2.
Analysis of flavonoids and isoflavones in human urine
As a typical application of the developed gradient reversed-phase UHPLC method, urinary excretion of flavonoids and isoflavones from healthy volunteers was investigated. To further evaluate the applicability of the method, a subset of urine samples from an intervention study that involved patients on a diet high in flavonoid content, and with supplementation of isoflavones was analysed. The pharmaceutical preparations that were taken by the volunteers, contained GT, DA, GLY, and RUT.
Enzymatic hydrolysis was used for preparation of human urine samples containing isoflavones and flavonoids. In this work, the glycosides were hydrolyzed with pure β-glucuronidase and sulfatase (crude preparation from H. pomatia) in acetate buffer (pH 4.66), in order to release the aglycones [7]. The concentrations of analysed compounds increased after hydrolysis, because of conversion of the glycosides to the aglycone forms. Flavonoids and isoflavones are analysed as aglycones because of the high cost of the reference compounds or because some of them are not commercially available.
Flavonoid and isoflavone peak identification was achieved by comparison of retention times and by use of the standard addition method. UV detection was chosen for quantitative analysis because the detector was sufficiently sensitive and accurate for the prominent flavonoids and isoflavones in urine samples. The flavonoid and isoflavone content of the urine samples was determined using calibration curves.
Analysis of the 24-h urinary excretion of GLY, DA, and GT demonstrated that the cumulative amounts gradually increased during the collection period after oral administration of these isoflavones to the subject. The mean urine concentration–time profiles of GLY, DA, and RUT are shown in Fig. 4. The flavonoid and isoflavone concentrations reached a maximum between 5 and 8 h after dosing with a level of 5482.33 ± 10.78 ng mL−1 for GLY, 228.03 ± 5.34 ng mL−1 for DA, and 780.18 ± 10.39 ng mL−1 for RUT. Assuming the mean 24-h urine volume is 1.4 L [17], the approximate average daily output is 1.73 mg, 0.14 mg, and 0.19 mg of GLY, DA and RUT, respectively. The percentage of GLY, DA, and RUT excreted in urine within 24 h after ingestion of the tablets was 30%, 17%, and 1%, respectively, of the administered dose. The corresponding pharmacokinetic data for GLY, DA, and RUT are presented in Table 3.
The urine samples were also tested for (±)-CA, (−)-EC, QUR, and HST, which are components of a diet rich in fruits, vegetables, tea, and wine. The mean concentrations of these flavonoids were: 5427.34 ng mL−1 for (±)-CA, 2724.26 ng mL−1 for (−)-EC, and 73.57 ng mL−1 for QUR. HST was detected in urine, but not quantified (nq). The relationship between the concentrations of these compounds and the collection times of the urine samples is not shown.
An example chromatogram obtained from a urine sample is presented in Fig. 5. Based on the results, the sensitivity is sufficient to determine the presence of flavonoids and isoflavones in urine without complicated pre-treatment of the samples. Dilution studies revealed that urine samples with analyte concentrations outside the linear range of the calibration curves could be successfully analysed after dilution of the urine sample with organic solvents.
Chromatogram obtained by use of the UHPLC method from a urine sample containing flavonoids (6.38 μg mL−1 (±)-CA, 5.24 μg mL−1 (−)-EC, 0.79 μg mL−1 RUT, 0.15 μg mL−1 QUR, HST (nq)) and isoflavones (5.48 μg mL−1 GLY, 0.19 μg mL−1 DA). The sample was obtained from a healthy volunteer on a diet rich in flavonoids and orally administered tablets containing the flavonoids and isoflavones (collected 5 h after administration of the tablets)
HPLC–ESI-MS–MS was used for confirm the presence of the compounds because of its high selectivity in the detection of flavonoids and isoflavones in complex biological matrices.
Conclusion
In the work discussed in this paper, a simple gradient reversed-phase UHPLC method was developed and validated for simultaneous identification and determination of flavonoids and isoflavones in human urine. The UHPLC method was developed for quantification of the flavonoids (±)-catechin, (−)-epicatechin, rutin, hesperidin, neohesperidin, quercitrin, and hesperetin and the isoflavones genistein, daidzein, glycitin, puerarin, and biochanin A. This method enables rapid, accurate, and extensive analysis of flavonoids and isoflavones, utilizing a simple but effective sample-preparation procedure; thus, it enables large-scale epidemiological studies to be conducted. Hydrolysis of urine samples with enzyme resulted in high recoveries of flavonoids and isoflavones. The developed method can be applied to monitoring the concentration of isoflavones and flavonoids in humans without requiring hospitalization, because determination of these compounds in urine does not require invasive sample collection.
Finally, this new UHPLC method also has potential applicability to bioequivalence studies of flavonoids and isoflavones and will be useful for tracing the therapeutic effects of medications for certain diseases.
References
Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Dominguez H, Núñez MJ, Parajó JC (2001) Food Chem 72:145–171
Getoff N (2007) Radiat Phys Chem 76:1577–1586
Xi H, Akishita M, Nagai K, Yu W, Hasegawa H, Eto M, Kozaki K, Toba K (2007) Atherosclerosis 191:281–289
Havsteen BH (2002) Pharmacol Ther 96:67–202
Owolabi MA, Coker HAB, Jaja SI (2007) J Nat Med 61:200–204
Wang FM, Yao TW, Zeng S (2003) J Pharm Biomed Anal 33:317–321
Kanaze FI, Kokkalou E, Georgarakis M, Niopas I (2004) J Chromatogr B 801:363–367
Yang GJ, Liu P, Qu XL, Xu MJ, Qu QS, Wang CY, Hu XY, Wang ZY (2007) J Chromatogr B 856:222–228
Ishii K, Furuta T, Kasuya Y (2003) J Chromatogr B 794:49–56
Lai X, Zhao Y, Liang H, Bai Y, Wang B, Guo D (2007) J Chromatogr B 852:108–114
Chu KO, Wang CC, Rogers MS, Choy KW, Pang CP (2004) Anal Chim Acta 510:69–76
Simonetti P, Gardana TC, Riso P, Mauri P, Pietta P, Porrini M (2005) Nutr Res 25:717–726
Wang L, Morris ME (2005) J Chromatogr B 821:194–201
Ishii K, Furuta T, Kasuya Y (2001) J Chromatogr B 759:161–168
Bolarinwa A, Linseisen J (2005) J Chromatogr B 823:143–151
Setchell KD, Faughnan MS, Avades T, Zimmer-Nechemias MF, Brown NM, Wolfe BE, Brashear WT, Desai P, Oldfield MF, Botting NP, Cassidy A (2003) Am J Clin Nutr 77:411–419
Grace PB, Taylor JI, Botting NP, Fryatt T, Oldfield MF, Bingham SA (2003) Anal Biochem 315:114–121
Maubach J, Bracke ME, Heyerick A, Depypere HT, Serreync RF, Mareelb MM, Keukeleire DD (2003) J Chromatogr B 784:137–144
Ma Z, Wu Q, Lee DYW, Tracy M, Lukas SE (2005) J Chromatogr B 823:108–114
Yan B, Xing D, Ding Y, Tao J, Du L (2005) J Pharm Biomed Anal 37:297–301
Nurmi T, Adlercreutz H (1999) Anal Biochem 274:110–117
Klejdus B, Vacek J, Adam V, Zehnálek J, Kizek R, Trnková L, Kubáň V (2004) J Chromatogr B 806:101–111
Prasain JK, Jones K, Brissie N, Moore R, Wyss JM, Barnes S (2004) J Agric Food Chem 52:3708–3712
Holder CL, Churchwell MI, Doerge DR (1999) J Agric Food Chem 47:3764–3770
Grace PB, Mistry NS, Carter MH, Leathem AJC, Teale P (2007) J Chromatogr B 853:138–146
Grace PB, Taylor JI, Botting NP, Fryatt T, Oldfield MF, Al-Maharik N, Bingham SA (2003) Rapid Commun Mass Spectrom 17:1350–1357
Fang N, Yu S, Badger TM (2002) J Agric Food Chem 50:2700–2707
Adlercreutz H, Fotsis T, Kurzer MS, Wahala K, Makela T, Hase T (1995) Anal Biochem 225:101–108
Cueto-Rojas HF, Pérez NO, Pérez-Sánchez G, Ocampo-Juárez I, Medina-Rivero E (2010) J Chromatogr B 878:1019–1023
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Focus on Analytical Science in Poland (VIIIth Polish Conference on Analytical Chemistry) with Guest Editor Pawel Koscielniak.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 63.2 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Baranowska, I., Magiera, S. Analysis of isoflavones and flavonoids in human urine by UHPLC. Anal Bioanal Chem 399, 3211–3219 (2011). https://doi.org/10.1007/s00216-010-4206-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4206-6