Abstract
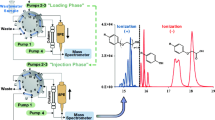
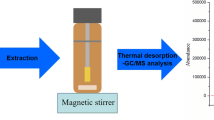
A procedure for the determination of seven parabens (esters of 4-hydroxybenzoic acid), including the distinction between branched and linear isomers of propyl- and butyl-parabens and triclosan in water samples, was developed and evaluated. The procedure includes in-sample acetylation-non-porous membrane-assisted liquid–liquid extraction and large volume injection–gas chromatography–ion trap–tandem mass spectrometry. Different derivatisation strategies were considered, i.e. post-extraction silylation with N-methyl-N-(tert-butyldimethylsilyl)-trifluoroacetamide and in situ acylation with acetic anhydride (Ac2O) and isobutylchloroformate. Moreover, acceptor solvent and the basic catalyser of the acylation reaction were investigated. Thus, in situ derivatisation with Ac2O and potassium hydrogenphosphate (as basic catalyser) was selected. Potassium hydrogenphosphate overcomes some drawbacks of other basic catalysers, e.g. toxicity and bubble formation, while leads to higher responses. Subsequently, other experimental variables affecting derivatisation–extraction yield such as pre-stirring time, salt addition and volume of Ac2O were optimised by an experimental design approach. Under optimised conditions, the proposed method achieved detection limits from 0.1 to 1.4 ng L−1 for a sample volume of 18 mL and extraction efficiencies, estimated by comparison with liquid–liquid extraction, between 46% (for methyl- and ethyl-parabens) and 110% (for benzylparaben). The reported sample preparation approach is free of matrix effects for parabens but affected for triclosan with a reduction of ≈ 40% when wastewater samples are analysed; therefore, both internal and external calibration can be used as quantification techniques for parabens, but internal standard calibration is mandatory for triclosan. The application of the method to real samples revealed the presence of these compounds in raw wastewater at concentrations up to 26 ng mL−1, the prevalence of the linear isomer of propylparaben (n-PrP), and the coexistence of the two isomers of butylparaben (i-BuP and n-BuP) at similar levels.







Similar content being viewed by others
References
Official Journal of the European Communities, OJ L 262, 27.9.1976, p. 169, 1976
Peck AM (2006) Anal Bioanal Chem 386:907–939
Crofton KM, Paul KB, De Vito MJ, Hedge JM (2007) Environ Toxicol Pharmacol 24:194–197
Silva E, Rajapakse N, Kortenkamp A (2002) Environ Sci Technol 36:1751–1756
Darbre PD, Aljarrah A, Miller WR, Coldham NG, Sauer MJ, Pope GS (2004) J Appl Toxicol 24:5–13
Canosa P, Morales S, Rodríguez I, Rubí E, Cela R, Gómez M (2005) Anal Bioanal Chem 383:1119–1126
Lores M, Llompart M, Sánchez-Prado L, García-Jares C, Cela R (2005) Anal Bioanal Chem 381:1294–1298
Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstein A, Cunningham V (2002) Environ Toxicol Chem 21:1338–1349
Canosa P, Rodríguez I, Rubí E, Bollaín MH, Cela R (2006) J Chromatogr A 1124:3–10
Lee HB, Peart TE, Svoboda ML (2005) J Chromatogr A 1094:122–129
Singer H, Müller S, Tixier C, Pillonel L (2002) Environ Sci Technol 36:4998–5004
Canosa P, Rodríguez I, Rubí E, Cela R (2005) J Chromatogr A 1072:107–115
González-Mariño I, Quintana JB, Rodríguez I, Cela R (2009) Rapid Commun Mass Spectrom 23:1756–1766
Regueiro J, Becerril E, García-Jares C, Llompart M (2009) J Chromatogr A 1216:4693–4702
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2008) Water Res 42:3498–3518
Benijts T, Lambert W, De Leenheer A (2004) Anal Chem 76:704–711
Pedrouzo M, Borrull F, Marcé RM, Pocurull E (2009) J Chromatogr A 1216:6994–7000
Saraji M, Mirmahdieh S (2009) J Sep Sci 32:988–995
Regueiro J, Llompart M, Psillakis E, García-Monteagudo JC, García-Jares C (2009) Talanta 79:1387–1397
Montes R, Rodríguez I, Rubí E, Cela R (2009) J Chromatogr A 1216:205–210
Moeder M, Lange F (2007) LC-GC Eur 20:97–103
Rodil R, Schrader S, Moeder M (2009) J Chromatogr A 1216:4887–4894
Wells RJ (1999) J Chromatogr A 843:1–18
Llompart M, Lourido M, Landín P, García-Jares C, Cela R (2002) J Chromatogr A 963:137–148
Henriksen T, Svensmark B, Lindhardt B, Juhler RK (2001) Chemosphere 44:1531–1539
Rodríguez I, Llompart MP, Cela R (2000) J Chromatogr A 885:291–304
Einsle T, Paschke H, Bruns K, Schrader S, Popp P, Moeder M (2006) J Chromatogr A 1124:196–204
Hauser B, Popp P, Kleine-Benne E (2002) J Chromatogr A 963:27–36
Quintana JB, Reemtsma T (2006) J Chromatogr A 1124:22–28
Quintana JB, Rodil R, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D (2007) Anal Bioanal Chem 388:1283–1293
Pizarro C, González-Saiz JM, Pérez-Del-Notario N (2006) J Chromatogr A 1132:8–14
Lewis GA, Mathieu D, Phan-Tan-Luu R (1999) Pharmaceutical experimental design in drugs. Marcel Dekker, New York
Acknowledgements
This research was funded by the Spanish Ministry of Science and Innovation (Ministerio de Ciencia e Innovación) and FEDER funds: project no. CTQ2009-08377 and “Acciones Integradas” DE2009-0020. RR and JBQ extend their gratitude to the Spanish Ministry of Science and Innovation (Ramón y Cajal research program). IGM acknowledges the Spanish Ministry of Education (Ministerio de Educación) for her FPU grant. Finally, we are indebted to Aquagest for kindly providing access to wastewater samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villaverde-de-Sáa, E., González-Mariño, I., Quintana, J.B. et al. In-sample acetylation-non-porous membrane-assisted liquid–liquid extraction for the determination of parabens and triclosan in water samples. Anal Bioanal Chem 397, 2559–2568 (2010). https://doi.org/10.1007/s00216-010-3789-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3789-2