Abstract
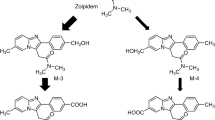
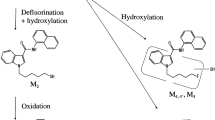
The objective of the present study was to investigate mesocarb metabolism in humans. Samples obtained after administration of mesocarb to healthy volunteers were studied. The samples were extracted at alkaline pH using ethyl acetate and salting-out effect to recover metabolites excreted free and conjugated with sulfate. A complementary procedure was applied to recover conjugates with glucuronic acid or with sulfate consisting of the extraction of the urines with XAD-2 columns previously conditioned with methanol and deionized water; the columns were then washed with water and finally eluted with methanol. In both cases, the dried extracts were reconstituted and analyzed by ultra-performance liquid chromatography–tandem mass spectrometry. Chromatographic separation was carried out using a C18 column (100 mm × 2.1 mm i.d., 1.7 µm particle size) and a mobile phase consisting of water and acetonitrile with 0.01% formic acid with gradient elution. The chromatographic system was coupled to a mass spectrometer with an electrospray ionization source working in positive mode. Metabolic experiments were performed in multiple-reaction monitoring mode by monitoring one transition for each potential mesocarb metabolite. Mesocarb and 19 metabolites were identified in human urine, including mono-, di-, and trihydroxylated metabolites excreted free as well as conjugated with sulfate or glucuronic acid. All metabolites were detected up to 48 h after administration. The structures of most metabolites were proposed based on data from reference standards available and molecular mass and product ion mass spectra of the peaks detected. The direct detection of mesocarb metabolites conjugated with sulfate and glucuronic acid without previous hydrolysis has been described for the first time. Finally, a screening method to detect the administration of mesocarb in routine antidoping control analyses was proposed and validated based on the detection of the main mesocarb metabolites in human urine (p-hydroxymesocarb and p-hydroxymesocarb sulfate). After analysis of several blank urines, the method demonstrated to be specific. Extraction recoveries of 100.3 ± 0.8 and 105.9 ± 10.8 (n = 4), and limits of detection of 0.5 and 0.1 ng mL−1 were obtained for p-hydroxymesocarb sulfate and p-hydroxymesocarb, respectively. The intra- and inter-assay precisions were estimated at two concentration levels, 50 and 250 ng mL−1, and relative standard deviations were lower than 15% in all cases (n = 4).







Similar content being viewed by others
References
World Anti-Doping Agency (WADA). The World Anti Doping Code. The 2009 Prohibited List International Standard. (2008) http://www.wada-ama.org/rcontent/document/2009_Prohibited_List_ENG_Final_20_Sept_08.pdf
Polgár M, Vereczeley L, Szporny L, Czira G, Tamás J, Gács-Baitz E, Holly S (1979) Xenobiotica 9:511–520
Ventura R, Nadal T, Alcalde P, Segura J (1993) J Chromatogr 647:203–210
Ventura R, Nadal T, Alcalde P, Pascual JA, Segura J (1993) J Chromatogr A 655:233–242
Thieme D, Grobe J, Lang R, Mueller RK (1994) In: Donike M, Greyer H, Gotzmann A, Mareck-Engelke U (eds) Proceedings of the 12th Cologne Workshop on Dope Analysis. Cologne, Germany, pp275–285
Appolonova SA, Shpak AV, Semenov VA (2004) J Chromatogr B 800:281–289
Shpak AV, Appolonova SA, Semenov VA (2005) J Chromatogr Sci 43:11–21
Vahermo M, Suominen T, Leinonen A, Yli-Kauhaluoma J (2009) Arch Pharm Chem Life Sci 342:201–209
Pyo H, Park SJ, Park J, Yoo JK, Yoon B (1996) J Chromatogr B 687:261–269
Deventer K, Van Eenoo P, Delbeke PT (2005) Rapid Commun Mass Spectrom 19:90–98
Kang MJ, Hwang YH, Lee W, Kim DH (2007) Rapid Commun Mass Spectrom 21:252–264
Mazzarino M, Botrè F (2006) Rapid Commun Mass Spectrom 26:3465–3476
Ventura R, Roig M, Monfort N, Sáez P, Bergés R, Segura J (2008) Eur J Mass Spectrom 14:191–200
Kolmonen M, Leinonen A, Pelander A, Ojanperä I (2007) Anal Chim Acta 585:94–102
Kolmonen M, Leinonen A, Kuuranne T, Pelander A, Ojanperä I (2009) Drug Test Anal 1:250–266
Lee J, Jeung H, Kim K, Lho D (1999) J Mass Spectrom 34:1079–1086
Jiménez C, de la Torre R, Segura J, Ventura R (2006) Rapid Commun Mass Spectrom 20:858–864
Jiménez C, Ventura R, Segura J (2002) J Chromatogr B Analyt Technol Biomed Life Sci 767:341–351
World Anti-Doping Agency (WADA). Minimum required performance limits for detection of prohibited substances. WADA Technical Document TD2009MRPL (2009) http://www.wada-ama.org/rtecontent/document/MINIMUM_REQUIRED_PERFORMANCE_LEVELS_TD_v1_0_January_2009.pdf
Acknowledgments
The financial support received from Consell Català de l’Esport, Generalitat de Catalunya (Spain) and Ministerio de Educación y Ciencia (Spain; project number DEP2007-73224-C03-03) to R. V. and the World Anti-Doping Agency WADA (Grant 05D4JY/2005) and Research Foundation of Clinical Chemistry (Kliinisen kemian tutkimussäätiö, Helsinki, Finland) to J. Y.-K. is greatly acknowledged. The collaboration of Dr. Magí Farré to obtain administration study samples is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Screening method: MRM chromatograms of blank urine (top) and chromatograms of a quality control sample spiked with 50 ng mL-1 of p-hydroxymesocarb (2) and p-hydroxymesocarb sulfate (11) (bottom) (PDF 575 kb)
Rights and permissions
About this article
Cite this article
Gómez, C., Segura, J., Monfort, N. et al. Identification of free and conjugated metabolites of mesocarb in human urine by LC-MS/MS. Anal Bioanal Chem 397, 2903–2916 (2010). https://doi.org/10.1007/s00216-010-3756-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3756-y