Abstract
A colloidal synthesis method was developed to produce face centered cubic (fcc) Cu nanoparticles in the presence of surfactants in an organic solvent under an Ar environment. Various synthetic conditions were explored to control the size of the as-prepared nanoparticles by changing the precursor, varying the amount of surfactants, and tuning the reaction temperature. Transmission electron microscopy (TEM), selected-area electron diffraction, and high-resolution TEM were used as the main characterization tools. Upon exposure to air, these nanoparticles are oxidized at different levels depending on their sizes: (1) an inhomogeneous layer of fcc Cu2O forms at the surface of Cu nanoparticles (about 30 nm); (2) Cu nanoparticles (about 5 nm) are immediately oxidized into fcc Cu2O nanoparticles (about 6 nm). The occurrence of these different levels of oxidization demonstrates the reactive nature of Cu nanoparticles and the effect of size on their reactivity. Furthermore, utilization of their chemical reactivity and conversion of spherical Cu nanoparticles into CuS nanoplates through the nanoscale Kirkendall effect were demonstrated. The oxidization and sulfidation of Cu nanoparticles were compared. Different diffusion and growth behaviors were involved in these two chemical transformations, resulting in the formation of isotropic Cu2O nanoparticles during oxidization and anisotropic CuS nanoplates during sulfidation.

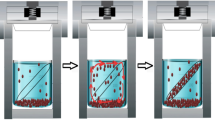
Transmission electron microscopy images of Cu nanoparticles (left), Cu2O nanoparticles (middle), and CuS nanoplates (right)








Similar content being viewed by others
References
Yin Y, Alivisatos AP (2005) Nature 437:664–670
Tao AR, Habas S, Yang PD (2008) Small 4:310–325
Xia Y, Xiong YJ, Lim B, Skrabalak SE (2009) Angew Chem Int Ed 48:60–103
Yin YD, Rioux RM, Erdonmez CK, Hughes S, Somorjai GA, Alivisatos AP (2004) Science 304:711–714
Yin YD, Erdonmez CK, Cabot A, Hughes S, Alivisatos AP (2006) Adv Funct Mater 16:1389–1399
Cabot A, Puntes VF, Shevchenko E, Yin Y, Balcells L, Marcus MA, Hughes SM, Alivisatos AP (2007) J Am Chem Soc 129:10358–10360
Cabot A, Smith RK, Yin YD, Zheng HM, Reinhard BM, Liu HT, Alivisatos AP (2008) ACS Nano 2:1452–1458
Lewinski N, Colvin V, Drezek R (2008) Small 4:26–49
Grassian VH (2008) J Phys Chem C 112:18303–18313
Lisiecki I, Pileni MP (1993) J Am Chem Soc 115:3887–3896
Tanori J, Pileni MP (1997) Langmuir 13:639–646
Salzemann C, Lisiecki I, Brioude A, Urban J, Pileni MP (2004) J Phys Chem B 108:13242–13248
Salzemann C, Lisiecki L, Urban J, Pileni MP (2004) Langmuir 20:11772–11777
Murray CB, Kagan CR, Bawendi MG (2000) Annu Rev Mater Sci 30:545–610
Yin M, Wu CK, Lou YB, Burda C, Koberstein JT, Zhu YM, O'Brien S (2005) J Am Chem Soc 127:9506–9511
Mott D, Galkowski J, Wang LY, Luo J, Zhong CJ (2007) Langmuir 23:5740–5745
Cheng GJ, Carter JD, Guo T (2004) Chem Phys Lett 400:122–127
Cheng GJ, Romero D, Fraser GT, Walker ARH (2005) Langmuir 21:12055–12059
Cheng GJ, Dennis CL, Shull RD, Walker ARH (2007) Langmuir 23:11740–11746
Cheng GJ, Shull RD, Walker ARH (2009) J Magn Magn Mater 321:1351–1355
Cheng GJ, Dennis CL, Shull RD, Walker ARH (2009) Cryst Growth Des 9:3714–3720
Cheng GJ, Puntes VF, Guo T (2006) J Colloid Interface Sci 293:430–436
Bruneval F, Vast N, Reining L, Izquierdo M, Sirotti F, Barrett N (2006) Phys Rev Lett 97:267601
Gou LF, Murphy CJ (2003) Nano Lett 3:231–234
Zhao YX, Pan HC, Lou YB, Qiu XF, Zhu JJ, Burda C (2009) J Am Chem Soc 131:4253–4261
Wang ZL (2000) J Phys Chem B 104:1153–1175
Ding Y, Wang ZL (2004) J Phys Chem B 108:12280–12291
Lide DR (ed) (2008) CRC handbook of chemistry and physics, 88th edn. CRC, Boca Raton
Wang KJ, Li GD, Li JX, Wang Q, Chen JS (2007) Cryst Growth Des 7:2265–2267
Ghezelbash A, Korgel BA (2005) Langmuir 21:9451–9456
Goncalves AP, Lopes EB, Casaca A, Dias M, Almeida M (2008) J Cryst Growth 310:2742–2745
Li BX, Xie Y, Xue Y (2007) J Phys Chem C 111:12181–12187
Lofton C, Sigmund W (2005) Adv Funct Mater 15:1197–1208
Ascencio JA, Gutierrez-Wing C, Espinosa ME, Marin M, Tehuacanero S, Zorrilla C, Jose-Yacaman M (1998) Surf Sci 396:349–368
Hofmeister H (1998) Cryst Res Technol 33:3–25
Palkar VR, Ayyub P, Chattopadhyay S, Multani M (1996) Phys Rev B 53:2167–2170
Tang Y, Ouyang M (2007) Nat Mater 6:754–759
Acknowledgements
We thank Li-Chung Lai and Wen-An Chiou for their help with TEM measurements. We acknowledge the support of the Maryland NanoCenter and its NispLab. The NispLab is supported in part by the NSF as an MRSEC Shared Experimental Facility.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer
We identify certain commercial equipment, instruments, and materials in this article to specify adequately the experimental procedure. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Rights and permissions
About this article
Cite this article
Cheng, G., Hight Walker, A.R. Transmission electron microscopy characterization of colloidal copper nanoparticles and their chemical reactivity. Anal Bioanal Chem 396, 1057–1069 (2010). https://doi.org/10.1007/s00216-009-3203-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3203-0