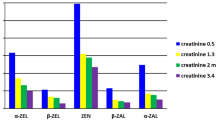
Abstract
Stilbenes and zeranol are nonsteroidal estrogenic growth promoters which are banned in the European Union (EU) for use in food-producing animals by Council Directive 96/22/EC. A liquid chromatography–tandem mass spectrometry (LC-MS/MS) method was developed for the screening and confirmation of stilbenes (diethylstilbestrol, dienestrol, hexestrol) and resorcylic acid lactones (zeranol and its metabolites taleranol and zearalanone as well as the mycotoxins α-zearalenol, β-zearalenol and zearalenone) in bovine urine. The method permits the confirmation and quantification of stilbenes and resorcylic acid lactones at levels below 1 µg L−1 and 1.5 µg L−1, respectively. The validation was carried out according to Commission Decision 2002/657/EC, Chap. 3.1.3 “alternative validation” by a matrix-comprehensive in-house validation concept. Decision limit CCα, detection capability CCβ, recovery, repeatabiliy, within-laboratory reproducibility and the uncertainty of measurement were calculated. Furthermore, a factorial effect analysis was carried out to identify factors that have a significant influence on the method. Factors considered to be relevant for the method in routine analysis (e.g. operator, matrix condition, storage duration of the extracts before measurement, different cartridge lots, hydrolysis conditions) were systematically varied on two levels. The factorial analysis showed that different cartridge lots, storage durations and matrix conditions can exert a relevant influence on the method.




Similar content being viewed by others
References
Council Directive 96/22/EC of 29 April 1996 concerning the prohibition on the use in stockfarming of certain substances having a hormonal or thyreostatic action and of beta-agonists (1996). Off J Eur Commun L125:3–9
Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products (1996). Off J Eur Commun L125:10–32
Regulation (EC) No 882/2004 of the European Parliament and of the Council of 29 April 2004 on official controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare rules (2004) Off J Eur Comm L165:1–141
Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (2002). Off J Eur Comm L221:8
Document SANCO 2726/2004 rev. 1 (2004) Guidelines for the implementation of Decision 2002/657/EC. http://ec.europa.eu/food/food/chemicalsafety/residues/guidelines_2002-657.pdf.
Community Reference Laboratories for Residues (2007) CRL guidance paper, 7 December 2007: CRLs view on state of the art analytical methods for national residue control plans. http://fis-vl.bund.de/Public/irc/fis-vl/Home/main (restricted access).
Kootstra PR, Zoontjes PW (2007) Multi-residue screening of a minimum package of anabolic steroids in urine with GC-MS. Anal Chim Acta 586:82–92
Impens S, Van Loco J, Degroodt JM, De Brabander H (2007) A downscaled multi-residue strategy for detection of anabolic steroids in bovine urine using gas chromatography tandem mass spectrometry (GC-MS3). Anal Chim Acta 586:43–48
Haedrich J, Faller C (2005) Ressourcenschonende Ermittlung robuster Leistungskenndaten: ein pragmatischer Validierungsansatz für Bestätigungsverfahren- Teil 1: Stilbene, natürliche und synthetische Steroidhormone. Deutsche Lebensmittel-Rundschau 101:525–533
Aschbacher PW, Thacker EJ (1974) Metabolic fate of oral diethylstilbestrol in steers. J Anim Sci 39:1185–1192
Kleinova M, Zöllner P, Kahlbacher H, Hochsteiner W, Lindner W (2002) Metabolic Profiles of the mycotoxin zearalenone and of the growth promoter zeranol in urine liver and muscle of heifers. J Agric Food Chem 50:4769–4776
Lone KP (1997) Natural sex steroids and their xenobiotic analogs in animal production: growth, carcass quality, pharmacokinetics, metabolism, mode of action, residues, methods and epidemiology. Crit Rev Food Sci Nutrit 37:93–209 (and references cited)
Chichila TMP, Silvestre D, Covey TR, Henion JD (1988) Distribution of zeranol in bovine tissues determined by selected ion monitoring capillary gas chromatography/mass spectrometry. J Anal Toxicol 12:310–318
Miles CO, Erasmuson AF, Wilkins AL, Towers NR, Smith BL, Garthwaite I, Scahill BG, Hansen RP (1996) Ovine metabolism of zearalenone to α-zearalanol (zeranol). J Agric Food Chem 44:3244–3250
Kennedy DG, McEvoy JDG, Blanchflower WJ, Hewitt SA, Cannavan A, McCaughey WJ, Elliot CT (1995) Possible naturally occurring zeranol in bovine bile in Northern Ireland. J Vet Med B 42:509–512
Kennedy DG, Hewitt SA, McEvoy JDG, Currie JW, Cannavan A, Blanchflower WJ, Elliot CT (1998) Zeranol is formed from Fusarium spp. toxins in cattle in vivo. Fodd Add Cont 15:393–400
Zöllner P, Jodlbauer J, Kleinova M, Kahlbacher H, Kuhn T, Hochsteiner W, Lindner W (2002) Concentration levels of zearalanone and its metabolites in urine, muscle, tissue and liver samples of pigs fed with mycotoxin-contaminated oats. J Agric Food Chem 50:2494–2501
Launay FM, Young PB, Sterk SS, Blokland MH, Kennedy DG (2004) Confirmatory assay for zeranol, taleranol and the Fusarium spp. toxins in bovine urine using liquid chromatography-tandem mass spectrometry. Food Add Contam 21:52–62
van Bennekom EO, Brouwer L, Laurant EHM, Hooijerink H, Nielen MWF (2002) Confirmatory analysis method for zeranol, its metabolites and related mycotoxins in urine by liquid chromatography-negative ion electrospray tandem mass spectrometry. Anal Chim Acta 473:151–160
Jülicher B, Gowik P, Uhlig S (1998) Assessment of detection methods in trace analysis by means of a statistically based in-house validation concept. Analyst 123:173–179
Jülicher B, Gowik P, Uhlig S (1999) A top-down in-house validation based approach for the investigation of the measurement uncertainty using fractional factorial experiments. Analyst 124:537–545
Uhlig S, Gowik P, Radeck W (2003) Performance of a matrix-comprehensive in-house validation study by means of an especially designed software. Anal Chim Acta 483:351–362
Bock C, Gowik P, Stachel C (2007) Matrix-comprehensive in-house validation and robustness check of a confirmatory method for the determination of four nitrofuran metabolites in poultry muscle and shrimp by LC-MS/MS. J Chrom B 856:178–189
InterValplus/ OptiVal (2008) Software for the method optimisation and validation. quo data GmbH, Dresden, Germany. http://www.quodata.de. Accessed 6 Feb 2008
Lea AR, Kayaba WJ, Hailey DM (1979) Analysis of diethylstilbestrol and its impurities in tablets using reversed-phase high-performance liquid chromatography. J Chrom 177:61–68
Hong X, Long G, Jia H, Anqing L, Danzhou T (2007) Simultaneous determination of residual stilbenes and stilbene metabolites in animal tissue by liquid chromatography-tandem mass spectrometry. J Chrom B 852:529–533
EURACHEM (2002) EURACHEM/CITAC Guide, 2nd edn. ISBN 0948926 15 5
Gowik P, Polzer J, Uhlig S (2007) Reference material in residue control: assessment of matrix effects. Accred Qual Assur 12:161–166
Acknowledgements
The authors wish to thank Mrs. M. Grunow and Mr. S. Zimmermann for their technical assistance in the in-house validation study. Dr. Frank Hamann is acknowledged for making available the samples of bovine urine. Dr. Steffen Uhlig is acknowledged for helpful discussions on statistical issues.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, K., Stachel, C. & Gowik, P. Development and in-house validation of an LC-MS/MS method for the determination of stilbenes and resorcylic acid lactones in bovine urine. Anal Bioanal Chem 391, 1199–1210 (2008). https://doi.org/10.1007/s00216-008-1943-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-1943-x