Abstract
Hydroxymethylfurfural (HMF), a well-known heterocyclic Maillard reaction product, has often been studied for its potential toxic, mutagenic, and carcinogenic effects. Recent clinical studies, however, have strongly suggested that HMF might have exciting antitumor potential. We report on the development and validation of a bioanalytical assay for HMF that could be suitable as a basis for pharmacokinetic models in cancer patients. Two strategies were tested, i.e., direct and indirect methodologies. A direct isocratic LC determination at 283 nm was designed. Two indirect attempts involved derivatization coupled to HPLC-UV. It was possible to resolve the stereoisomers of the HMF derivative, and factors influencing their equilibrium ratio are discussed. HMF was extracted from the biomatrix by solid-phase extraction using different cartridges. A comparative study was made of the implemented methods as well as the extraction protocols. Both indirect assays proved to be more sensitive and were used to assess HMF quantitatively in human plasma. However, the newly introduced derivatization conditions led to the highest sensitivity with a LOD (S/N ratio = 3) of at least 2 pmol analyte on column. The assay selectivity was satisfactory in pre- and post-dose real samples. The mean recoveries of the assays were 79% and 89%, with acceptable accuracies and reproducibilities.

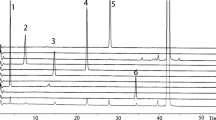
Schematic representation of hydroxymethylfurfural (HMF) in human plasma







Similar content being viewed by others
References
Nassberger L (1990) Hum Exp Toxicol 9:211–214
Ulbricht RJ, Northup SJ, Thomas JA (1984) Fundam Appl Toxicol 4:843–853
Rasmussen A, Hessov I, Bojsen-Moller M (1982) Acta Pharmacol Toxicol 50:81–84
Shinohara K, Kim E-H, Omura H (1986) Dev Food Sci 13:353–362
Kim SB, Hayase F, Kato H (1987) Mutat Res 177:9–15
Surh YJ, Tannenbaum SR (1994) Chem Res Toxicol 7:313–318
Janzowski C, Glaab V, Samimi E, Schlatter J, Eisenbrand G (2000) Food Chem Toxicol 38:801–809
Archer MC, Bruce WR, Chan CC, Corpet DE, Medline A, Roncucci L, Stamp D, Zhang XM (1992) Environ Health Persp 98:195–197
Zhang XM, Chan CC, Stamp D, Minkin S, Archer MC, Bruce WR (1993) Carcinogenesis 14:773–775
Miyakawa Y, Nishi Y, Kato K, Sato K, Takahashi M, Hayashi Y (1991) Carcinogenesis 12:1169–1173
Uckun FM, Shyi-Tai Jan M (2003) US Patent 6,258,841 B1
Ying D, Kevin P, Weihan Z, Xiaoqiang Y, Jianrong H (2005) US Patent 2005 124684 A1
Herwig R (2006) Pilotstudie KARAL- Monochemotherapien. Austrian Patent 393221 B
Rufian-Henares JA, Delgado-Andrade C, Morales FJ (2006) J AOAC Int 89:161–165
Gökmen V, Acar J (1999) J Chromatogr A 847:69–74
Menez JF, Berthou F, Meskar A, Picart D, Le Bras R, Bardou LG (1984) J Chromatogr A 297:339–350
Sampietro T, Lenzi S, Giampietro O, Cecchetti P, Masoni A, Navalesi R (1987) Clin Physiol Biochem 5:49–56
Lo Coco F, Valentini C, Novelli V, Ceccon L (1996) J Chromatogr A 749:95–102
Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A (2000) Pharm Res 17:1551–1557
Wieling J, Hendriks G, Tamminga WJ, Hempenius J, Mensink CK, Oosterhuis B, Jonkman JHG (1996) J Chromatogr A 730:381–394
Dadgar D, Burnett PE, Choc MG, Gallicano K, Hooper JW (1995) J Pharm Biomed Anal 13:89–97
Bressole F, Bromet-Petit M, Audran M (1996) J Chromatogr B 686:3–10
Jencks WP (1959) J Am Chem Soc 81:475–481
Ragno M (1945) Gazz Chim Ital 75:175–185
Legradi L (1971) Mikrochim Acta 2:380–383
Uchiyama S, Ando M, Aoyagi S (2003) J Chromatogr A 996:95–102
Tayyari SM, Speakman JL, Arnold MB, Cai W, Behforouz M (1998) J Chem Soc Perkin Trans 2:2195–2200
Uchiyama S, Matsushima E, Aoyagi S, Ando M (2004) Anal Chim Acta 523:157–163
Karabatsos GJ, Shapiro BL, Vane FM, Fleming JS, Ratka JS (1963) J Am Chem Soc 85:2784–2788
Shvo Y, Nahlieli A (1970) Tetrahedron Lett 49:4273–4274
Pichon R, Le Saint J, Courtot P (1981) Tetrahedron 37:1517–1524
Clarke LF, O’Sullivan F, Hegarty AF (1991) J Chem Soc Perkin Trans 2 1649–1652
Dadgar D, Burnett PE (1995) J Pharm Biomed Anal 14:23–31
Braddock LI, Garlow KY, Grim LI, Kirkpatrick AF, Pease SW, Pollard AJ, Price EF, Reissmann TL, Rose HA, Willard ML (1953) Anal Chem 25:301–306
Ramirez F, Kirby AF (1954) J Am Chem Soc 76:1037–1044
Karnes HT, Shiu G, Shah VP (1991) Pharm Res 8:421–426
Shah VP, Midha KK, Dighe S, McGilveray IJ, Skelly JP, Yacobi A, Layloff T, Viswanathan CT, Cook CE, McDowall RD, Pittman KA (1992) J Pharm Sci 81:309–312
Chapuzet E, Mercier N, Bervoas-Martin S, Boulanger B, Chevalier P, Chiap P, Grandjean D, Hubert P, Lagorce P, Lallier M, Laparra MC, Laurentie M, Nivet JC (1998) STP Pharm Prat 8:81–107
IUPAC (1997) Compendium of analytical nomenclature. Blackwell Science, Oxford
USEPA (2004) Revised assessement of detection and quatitation approaches. US Environmental Protection Agency Document EPA-821-B-04-005, Office of Science and Technology, Washington DC
Jellum E, Børresen HC, Eldjarn L (1973) Clin Chim Acta 47:191–201
Germond JE, Philippossian G, Richli U, Bracco I, Arnaud MJ (1987) J Toxicol Env Health 22:79–89
Parkash MK, Caldwell J (1994) Food Chem Toxicol 32:887–895
Grubbs FE (1969) Technometrics 11:1–21
Chow S-C, Liu J-P (1995) Statistical design and analysis in pharmaceutical sciences: validation and monographs, vol 143. Marcel Dekker, New York
Hartmann C, Smeyers-Verbeke J, Massart DL, Mcdowall RD (1998) J Pharm Biomed Anal 17:193–218
Hartmann C, Penninckx W, Vander Heyden Y, Vankeerberghen P, Massart DL, McDowall RD (1995) Experience with chromatographic methods-Europe. Blume HH, Midha KK (eds) Bio-international 2. Medpharm Scientific, Stuttgart
International Organization for Standardization (ISO) 5725-1 (1994) Accuracy (trueness and precision) of the results and methods of measurement, Part I: general principles and definitions. ISO, Geneva, Switzerland
Hubert P, Nguyen-Huu JJ, Boulanger B, Chapuzet E, Chiap P, Laurentie M, Mercier N, Muzard G, Nivet C, Valat L (2003) STP Pharm Prat 13:101–138
Satterthwaite FE (1946) Biometrics Bull 2:110–114
Food and Drug Administration (2001) Guidance for industry: bioanalytical method validation. US Food and Drug Administration, Washington DC, http://www.fda.gov/cder/guidance
International Conference on Harmonization (2005) Validation of analytical procedures: text and methodology Q2(R1), ICH harmonized tripartite guideline, http://www.ich.org/LOB/media/MEDIA417
Vander Heyden Y, Nijhuis A, Smeyers-Verbeke J, Vandegnste BGM, Massart DL (2001) J Pharm Biomed Anal 24:723–753
Acknowledgments
We are very grateful to C.Y.L. Pharmazeutika Gmbh especially Ch. Buecherl and P. Moser for the partial financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michail, K., Matzi, V., Maier, A. et al. Hydroxymethylfurfural: an enemy or a friendly xenobiotic? A bioanalytical approach. Anal Bioanal Chem 387, 2801–2814 (2007). https://doi.org/10.1007/s00216-007-1121-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1121-6