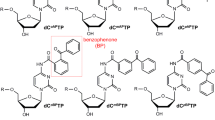
Abstract
The use of photolinkers (photoactivatable heterobifunctional crosslinkers) is a popular method to attach biomolecules to polymer surfaces. This study addresses the selection of photolinker and the adjustment of reaction conditions, such as the concentration of biomolecule applied, and irradiation time. The influence of these variables are investigated for four prominent photolinkers: ketyl-reactive benzophenone (BP) and anthraquinone (AQ), nitrene-reactive nitrophenyl azide (NPA), and carbene-reactive phenyl-(trifluoromethyl)diazirine (PTD). The influence of substrate material is discussed, and three different polymers served as representative substrates: poly(methyl methacrylate) (PMMA), polystyrene (PS), and a cycloolefin copolymer (COC). We compared the overall photolinking efficiency of all photolinkers with respect to the polymer substrate they are applied to, and we found considerable differences for certain photolinker/substrate combinations. Of all photolinkers and substrates tested, PTD as photolinker and COC as substrate showed the highest photolinking efficiencies and fastest reaction times. For this study DNA oligonucleotides were chosen as a model system of biomolecular probes, and fluorescence detection of DNA microarrays served as method of detection.





Similar content being viewed by others
Abbreviations
- AQ:
-
anthraquinone
- BP:
-
benzophenone
- BSA:
-
bovine serum albumin
- COC:
-
cycloolefin copolymer
- E PL :
-
photolinking efficiency
- NPA:
-
p-nitrophenyl azide
- PBS:
-
phosphate-buffered saline
- PL:
-
photolinker
- PMMA:
-
poly(methyl methacrylate)
- PS:
-
polystyrene
- PTD:
-
3-phenyl-3-(trifluoromethyl)diazirine
References
Wessa T, Rapp M, Sigrist H (1999) Colloids Surf B Biointerfaces 15:139–146
Ratner BD (1995) Biosens Bioelectron 10:797–804
Burke CS, Polerecky L, MacCraith BD (2004) Meas Sci Technol 15:1140–1145
Haynes CA, Norde W (1995) J Colloid Interface Sci 169:313–328
Buijs J, Norde W, Lichtenbelt JWT (1996) Langmuir 12:1605–1613
Chevrier D, Rasmussen SR, Guesdon J (1993) Mol Cell Probes 7:187–197
Sigrist H, Collioud A, Clemence J-F, Gao H, Luginbuehl R, Saenger M, Sundarababu G (1995) Opt Eng (Bellingham, Washington) 34:2339–2348
Holden MA, Jung S-Y, Cremer PS (2004) Anal Chem 76:1838–1843
Reichmuth P, Sigrist H, Badertscher M, Morf WE, de Rooij NF, Pretsch E (2002) Bioconjug Chem 13:90–96
Sabanayagam CR, Smith CL, Cantor CR (2000) Nucleic Acids Res 28:e33, ii–iv
Hermanson GT (ed) (1995) Bioconjugate Techniques. 1995
Brunner J (1993) Ann Rev Biochem 62:483–514
Dorman G, Prestwich GD (1994) Biochemistry 33:5661–5673
Yoshida E, Nakayama H, Hatanaka Y, Kanaoka Y (1990) Chem Pharm Bull 38:982–987
Groehn V, Frohlich L, Schmidt HHHW, Pfleiderer W (2000) Helv Chim Acta 83:2738–2750
Zotzmann J, Hennig L, Welzel P, Muller D, Schafer C, Zillikens S, Pusch H, Glitsch HG, Regenthal R (2000) Tetrahedron 56:9625–9632
Tyagi NK, Kinne RKH (2003) Anal Biochem 323:74–83
Clemence J-F, Ranieri JP, Aebischer P, Sigrist H (1995) Bioconjug Chem 6:411–417
Ma H, Davis RH, Bowman CN (2000) Macromolecules 33:331–335
Pouliquen L, Coqueret X (1996) Macromol Chem Phys 197:4045–4060
Schulz M, Matuschewski H, Wenschuh H, Thiele TA, Ulbricht M, Schedler U (2000) CLB Chem Lab Biotech 51:84–86
Kumar P, Agarwal SK, Gupta KC (2004) Bioconjug Chem 15:7–11
Koch T, Jacobsen N, Fensholdt J, Boas U, Fenger M, Jakobsen MH (2000) Bioconjug Chem 11:474–483
Liu X-h, Wang H-k, Herron JN, Prestwich GD (2000) Bioconjug Chem 11:755–761
Czarnecki J, Geahlen R, Haley B (1979) Methods Enzymol 56:642–653
Brunner J, Senn H, Richards FM (1980) J Biol Chem 255:3313–3318
Olszewski JD, Dorman G, Elliott JT, Hong Y, Ahern DG, Prestwich GD (1995) Bioconjug Chem 6:395–400
Chiara DC, Trinidad JC, Wang D, Ziebell MR, Sullivan D, Cohen JB (2003) Biochemistry 42:271–283
Ambroise Y, Pillon F, Mioskowski C, Valleix A, Rousseau B (2001) Eur J Org Chem 3961–3964
Ingenhorst G, Bindseil KU, Boddien C, Drose S, Gassel M, Altendorf K, Zeeck A (2001) Eur J Org Chem 4525–4532
Hori N, Iwai S, Inoue H, Ohtsuka E (1992) J Biol Chem 267:15591–15594
Wiedmann M, Kurzchalia TV, Bielka H, Rapoport TA (1987) J Cell Biol 104:201–208
Buchmueller KL, Hill BT, Platz MS, Weeks KM (2003) J Am Chem Soc 125:10850–10861
Weber T, Brunner J (1995) J Am Chem Soc 117:3084–3095
Nahar P, Naqvi A, Basir SF (2004) Anal Biochem 327:162–164
Guire P (1976) Methods Enzymol 44:280–288
Collioud A, Clemence JF, Saenger M, Sigrist H (1993) Bioconjug Chem 4:528–536
Gao H, Kislig E, Oranth N, Sigrist H (1994) Biotechnol Appl Biochem 20:251–263
Nassal M (1983) Liebigs Ann Chem 1510–1523
Acknowledgments
The presented work was part of the German national project “nanoMAP: modular application platform with integrated nanoliter dosage for highly parallel sample analysis” supported by the Federal Ministry of Education and Research (project no. 0312001B). The authors are grateful to the project partners microParts (Dortmund, Germany) for providing samples of polymer slides, and IMTEK (Freiburg, Germany) for their support regarding TopSpot technology. Daniela M. Dankbar was financially supported by the DFG Graduiertenkolleg “Analytische Chemie”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dankbar, D.M., Gauglitz, G. A study on photolinkers used for biomolecule attachment to polymer surfaces. Anal Bioanal Chem 386, 1967–1974 (2006). https://doi.org/10.1007/s00216-006-0871-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0871-x