Abstract
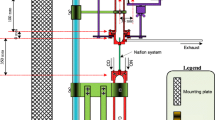
The use of natural antioxidants is of increasing importance in the human diet, because they are recognised as compounds essential to health which minimize or delay the aging process. Despite apparent simplicity, however, it is very difficult to measure and quantify such properties, for which a robust analytical method is required. Because oxidation usually is caused by the presence of OH· radicals, a new method involving the in-situ, vapour-phase generation of these radicals and their quantification in the presence and absence of potential antioxidant extracts has been developed. The oxidant atmosphere generated from hydrogen peroxide is carried by an air stream through an empty quartz chamber in which UV radiation promotes the formation of radicals by a photochemical reaction. The products then pass through a cartridge containing the essential oil, finally bubbling into an impinger containing an aqueous solution of salicylic acid, at pH 4.5, which reacts with the OH· radicals forming 2,5-dihydroxybenzoic acid. This solution is quantified by RP-HPLC using UV and fluorescence detectors connected in series. Detection and quantification limits for OH· radicals were approximately 0.01 pg g−1 air. Description and optimization of the method are discussed, as also is the antioxidant performance of an extract of ginger (Zingiber officinale R.), which reduced the oxidation process by up to 92%.





Similar content being viewed by others
References
Tovar L, Salafranca J, Sánchez C, Nerín C (2005) J Agric Food Chem 53:5270–5275
López P, Sánchez C, Batlle R, Nerín C (2005) J Agric Food Chem 53:6939–6946
Kranl K, Schlesier K, Bitsch R, Hermann H, Rohe M, Bohm V (2005) Food Chem 93:171–175
Mantle D, Anderton JG, Falkous G, Barnes M, Jones P, Perry EK (1998) Comp Biochem Phys B 121:385–391
Rey AI, Hopia A, Kivikari R, Kahkonen M (2005) LWT Food Sci Technol 38:363–370
Tang B, Zhang L, Geng Y (2005) Talanta 65:769–775
Salmon RA, Schiller CL, Harris GW (2004) J Atmos Chem 48:81–104
Librando V, Tringali G (2005) J Environ Manage 75:275–282
Li LX, Abe Y, Nagasawa Y, Kudo R, Usui N, Imai K, Mashino T, Mochizuki M, Miyata N (2004) Biomed Chromatogr 18:470–474
Mason TJ, Lorimer JP, Bates DM, Zhao Y (1994) Ultrason Sonochem 1:S91–S95
Benito Y, Arrojo S, Nerín C (2004) Unpublished results
Nuñez MT, Tapia V, Toyokuni S, Okada S (2001) Free Radical Res 34:57–68
Golden MC, Hahm SJ, Elessar RE, Saksonov S, Steinberg JJ (1998) Mycoses 41:97–104
Halliwell B, Kaur H, Ingelman-Sundberg M (1991) Free Radical Bio Med 10:439–441
Halliwell B, Grootveld M (1988) Method Biochem Anal 33:59–90
Dupont I, Berthou F, Bodenez P, Bardou L, Guirriec C, Stephan N, Dreano Y, Lucas D (1999) Drug Metab Dispos 27:322–326
Ingelman-Sundberg M, Kaur H, Terelius Y, Persson JO, Halliwell B (1991) Biochem J 276:753–757
Strolin-Benedetti M, Brogin G, Bani M, Oesch F, Hengstler JG (1999) Xenobiotica 29:1171–1180
Lang K, Brodilova J, Lunak S (1996) Collect Czech Chem C 61:1729–1737
Buxton GV, Langan JR, Smith JRL (1986) J Phys Chem 90:6309–6313
Daniel CH (2001) Quantitative chemical analysis, 5th edn. Reverté, Barcelona
Jen JF, Leu MF, Yang TC (1998) J Chromatogr A 796:283–288
Lang K, Wagnerova DM, Brodilova J (1994) Collect Czech Chem C 59:2447–2453
García I, Ortiz MC, Sarabia L, Vilches C, Gredilla E (2003) J Chromatogr A 992:11–27
Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, Bruni R (2005) Food Chem 91:621–632
Acknowledgements
This paper has been financed by the Project CAL03-080-from INIA – Ministerio de Ciencia y Tecnología, Spain. D. Pezo acknowledgements the grant obtained from SCH – University of Zaragoza.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pezo, D., Salafranca, J. & Nerín, C. Design of a method for generation of gas-phase hydroxyl radicals, and use of HPLC with fluorescence detection to assess the antioxidant capacity of natural essential oils. Anal Bioanal Chem 385, 1241–1246 (2006). https://doi.org/10.1007/s00216-006-0395-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0395-4